轻松扫码查看产品文档 | TCIMAIL No.197 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
CAS RN: 67-03-8 | 产品编码: T0181
Thiamine Hydrochloride

* 点击“查询”可查看预计发货日期,仅供参考。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
补充产品信息:
该产品名称和分子式此前为水合物形式,但由于其水合物没有专属的CAS RN,通常和非水合物共用。
为了避免不必要的误解,另外也因为水分可被认为是杂质,我们决定将水合物从产品名称中去除,同时移除分子式中的"xH2O"。
如果你在产品标签上看到的是之前的信息,请不要担心。产品品质将一如既往,请放心使用。
技术规格
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 98.0 area% |
| Purity(Argentometric Titration) | min. 98.0 %(calcd.on anh.substance) |
| Solubility in Water | almost transparency |
| Water | max. 5.0 % |
物性(参考值)
GHS
| 信号词 | 警告 |
| 危险性说明 | H303 : 吞咽可能有害。 |
| 防范说明 | P312 : 如感觉不适,呼叫急救中心/医生。 |
相关法规
| RTECS# | XI7350000 |
运输信息
| 监管条件代码(*) |
应用
Thiamine (Vitamin B1): One of Essential B Vitamins with Many Important Functions in All Tissues
Thiamine, also known as vitamin B1, is one of essential B vitamins that has many important functions in all tissues.1-3) Two types of water soluble thiamine salts, thiamine hydrochloride [T0181] and thiamine nitrate [T0182], are commonly used to products.4) Thiamine pyrophosphate (diphosphate) chloride [T0183] is the biologically active form of thiamine. In addition, thiamine hydrochloride is also used as an efficient catalyst for chemical reactions.7,8) For your reference, [A2633] is a key synthetic intermediate of thiamin. (The product is for research purpose only.)
References
- 1) Thiamin biosynthesis in prokaryotes (a review)
- 2) Molecular mechanisms of thiamine utilization (a review)
- 3) The structural and biochemical foundations of thiamin biosynthesis (a review)
- 4) Chemical stability and reaction kinetics of two thiamine salts (thiamine mononitrate and thiamine chloride hydrochloride) in solution
- 5) Analytical profile of thiamin hydrochloride (a review of the preparation, physical properties, metabolism, methods of determination, etc., of thiamin)
- 6) Determination of thiamine by high-performance liquid chromatography
- 7) Thiamine hydrochloride as a efficient catalyst for the synthesis of amidoalkyl naphthols
- 8) Thiamine hydrochloride (VB1): an efficient promoter for the one-pot synthesis of benzo[4,5]imidazo[1,2-a]pyrimidine and [1,2,4]triazolo[1,5-a]pyrimidine derivatives in water medium
应用
Synthesis of Formamide Derivatives Catalyzed by Vitamin B1 Hydrochloride
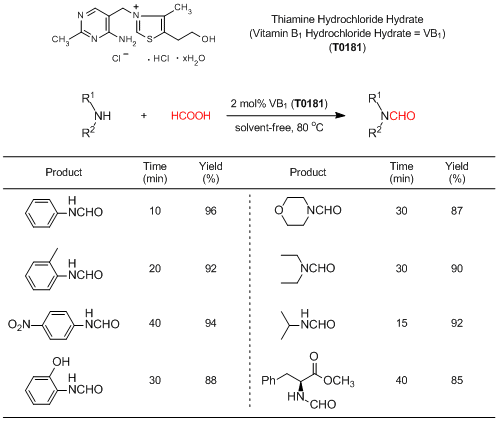
N-Formination of aniline using VB1 as a catalyst:
A mixture of aniline (5 mmol), formic acid (1 mL), and VB1 (0.1 mmol, 2 mol%) is heated at 80 °C under stirring for 10 minutes. After completion of the reaction as indicated by TLC, 20 mL of EtOAc is added and washed with 5% HCl aq., 5% Na2CO3 aq., and brine. Then, the organic layer is dried over MgSO4 and concentrated to afford N-formylaniline in 96% yield without further purification.
A mixture of aniline (5 mmol), formic acid (1 mL), and VB1 (0.1 mmol, 2 mol%) is heated at 80 °C under stirring for 10 minutes. After completion of the reaction as indicated by TLC, 20 mL of EtOAc is added and washed with 5% HCl aq., 5% Na2CO3 aq., and brine. Then, the organic layer is dried over MgSO4 and concentrated to afford N-formylaniline in 96% yield without further purification.
References
- A convenient one-pot synthesis of formamide derivatives using thiamine hydrochloride as a novel catalyst
应用
Thiamine Catalyzed Benzoin Condensation: Synthesis of Fluorobenzoins

Typical procedure : Synthesis of 4,4’-Difluorobenzoin
1,2-Bis(4-fluorophenyl)-2-hydroxyethanone is synthesized by condensation of 4-fluorobenzaldehyde (5.0 g, 40 mmol) in the presence of thiamine hydrochloride (0.67 g, 2.0 mmol) and sodium hydroxide (0.20 g, 5.0 mmol) in ethanol. The reaction is carried out at 50°C for 48 h and then the mixture is cooling on ice. A formed crude product is filtered and washed with ice cold 75% ethanol. The material is recrystallized from ethanol to give white crystals in 75% yield.
1,2-Bis(4-fluorophenyl)-2-hydroxyethanone is synthesized by condensation of 4-fluorobenzaldehyde (5.0 g, 40 mmol) in the presence of thiamine hydrochloride (0.67 g, 2.0 mmol) and sodium hydroxide (0.20 g, 5.0 mmol) in ethanol. The reaction is carried out at 50°C for 48 h and then the mixture is cooling on ice. A formed crude product is filtered and washed with ice cold 75% ethanol. The material is recrystallized from ethanol to give white crystals in 75% yield.
References
参考文献
文章/手册
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






