轻松扫码查看产品文档 | TCIMAIL No.197 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
CAS RN: 14024-18-1 | 产品编码: I0079
Tris(2,4-pentanedionato)iron(III)

* 点击“查询”可查看预计发货日期,仅供参考。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
技术规格
| Appearance | Red to Dark red to Brown powder to crystal |
| Purity(Chelometric Titration) | min. 98.0 % |
物性(参考值)
| 熔点 | 185 °C(dec.) |
| 水溶性 | 微溶 |
| 溶解性(可溶于) | 乙醇, 丙酮, 苯, 二氯甲烷, 甲苯, 氯仿 |
GHS
| 象形图 |

|
| 信号词 | 警告 |
| 危险性说明 | H302 : 吞咽有害。 H315 : 造成皮肤刺激。 H319 : 造成严重眼刺激。 |
| 防范说明 | P501 : 将内装物/容器送到批准的废物处理厂处理。 P270 : 使用本产品时不要进食、饮水或吸烟。 P264 : 作业后彻底清洗皮肤。 P280 : 戴防护手套/戴防护眼罩/戴防护面具。 P302 + P352 : 如皮肤沾染:用水充分清洗。 P337 + P313 : 如仍觉眼刺激:求医/就诊。 P305 + P351 + P338 : 如进入眼睛:用水小心冲洗几分钟。如戴隐形眼镜并可方便地取出,取出隐形眼镜。继续冲洗。 P362+P364 : 脱掉沾污的衣服,清洗后方可重新使用。 P332 + P313 : 如发生皮肤刺激:求医/就诊。 P301 + P312 + P330 : 如误吞咽:如感觉不适,呼叫急救中心/医生。漱口。 |
相关法规
| RTECS# | NO8960000 |
运输信息
| 监管条件代码(*) |
应用
2-PySO2CF2H : Bench-stable Difluoromethylating Agent for Catalytic Difluoromethylation

Reference
- Iron-Catalyzed Di?uoromethylation of Arylzincs with Di?uoromethyl 2?Pyridyl Sulfone
应用
Iron/dppbz-catalyzed Decarboxylative Couplings of Active Esters
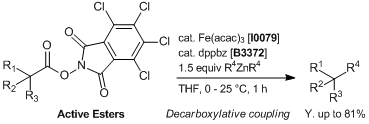
In a screwed-capped vial active ester (0.1 mmol), Fe(acac)3 (0.005 – 0.04 mmol) and dppbz (0.006 – 0.048 mmol) are weighed. The vial is then sealed, evacuated and back-filled with Ar. Then, 0.5 mL of distilled THF is added. The mixture is stirred for 5 minutes under Ar and diarylzinc reagent in THF (ca. 0.3 mol/L, 0.15 – 0.25 mmol) is added in one portion at 0 - 25 °C to the mixture and stirred at the same temperature for 1 h. After this time, the reaction is quenched with 1 mol/L HClaq, sat. NH4Claq or H2O and diluted with diethyl ether. The organic layer is separated and dried over Na2SO4 anhydrous, filtered and evaporated to dryness. Pure products are obtained after column chromatography or preparative TLC.
References
应用
Iron-Catalyzed ortho-Alkylation of Carboxamides

Typical procedure (R = benzyl): To Fe(acac)3 (17.1 mg, 0.05 mmol), dppe (29.9 mg, 0.075 mmol), and 3-methyl-N-(quinolin-8-yl)benzamide (131 mg, 0.5 mmol) are added THF (4 mL), and benzyl chloride (190 mg, 1.5 mmol). The mixture is then placed in an oil bath at 65 °C and PhMgBr (16% in THF, ca. 1 mol/L) (0.6 mL, 0.6 mmol) is added in a single portion. Upon completion of PhMgBr addition, the reaction mixture is stirred at 65 °C for 1 min. Then PhMgBr (16% in THF, ca. 1 mol/L) (1.3 mL, 1.3 mmol) is added dropwise at 65 °C for 9 min. The reaction mixture is stirred at 65 °C for 1 min and brought to rt. Subsequently the reaction mixture is quenched with a saturated solution of ammonium chloride (535 mg, 0.01 mol) and extracted with dichloromethane (3 x 3 mL). The combined organic extracts are dried over MgSO4, filtered and concentrated to give a residue. The residue is purified by silica gel flash chromatography (eluent: hexanes/EtOAc = 20:1) to give 2-benzyl-5-methyl-N-(quinolin-8-yl)benzamide (175 mg, 99% yield) as a white solid.
References
应用
Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
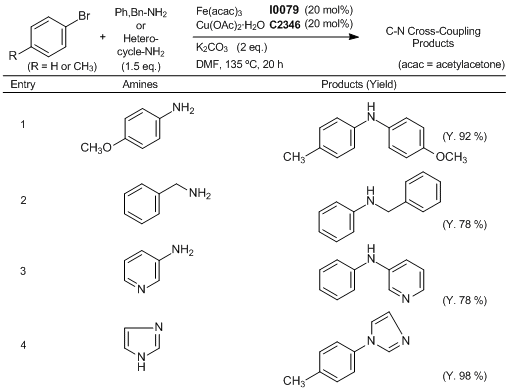
Typical procedure (Entry 1): A Schlenk tube is charged with p-anisidine (92.4 mg), K2CO3 (138 mg), Fe(acac)3 (34.4 mg), and Cu(OAc)2· H2O (20 mg). Under a nitrogen atmosphere, 4-bromotoluene (85.5 mg) is added followed by dry DMF (0.8 mL). The reaction mixture is then heated to 135 °C, and stirred for 20 h. The mixture is cooled to room temperature and diluted with diethyl ether. The resulting suspension is filtered through a celite pad and the filtrate is concentrated. The residue is purified by column chromatography on silica gel (eluent: petroleum ether/ethyl acetate) to give 4-methoxy-N-p-tolylaniline (98.1 mg, 92 %).
References
应用
Iron-Catalyzed Cross-Coupling Reaction

Reference
参考文献
TCIMail
[产品拾贝] 2-PySO2CF2H : 空气中稳定的二氟甲基化试剂,用于催化的二氟甲基化反应[研究论文] Fe或Ni催化下的活性酯脱羧C-C偶联反应
[研究论文] 铁催化酰胺邻位烷基化反应
[研究论文] Efficient Fe/Cu Co-Catalyzed C-N Cross-Coupling Reaction
产品文档 (部分产品的分析图谱无法提供,敬请谅解。)
化学品安全说明书(SDS)
请选择语言。
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
示例 CoA
可下载CoA示例。注:该示例不一定是最新批次的CoA。
目前没有该产品的 CoA 示例。
分析图谱
请输入批号
批号输入有误。请输入中横线前的4-5个字母数字字符。
很抱歉,您搜索的分析图谱无法提供。






