轻松扫码查看产品文档 | TCIMAIL No.197 已上新 | TCI试剂——品质可靠,值得信赖
订购方法?联系方式:021-67121386 / Sales-CN@TCIchemicals.com
Maximum quantity allowed is 999
请选择数量
CAS RN: 415918-91-1 | 产品编码: D4994
(R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine
![(R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine No-Image](/medias/D4994.jpg?context=bWFzdGVyfHJvb3R8Njc4OTd8aW1hZ2UvanBlZ3xhRFJoTDJneE5DODRPVEl4TkRZMU1EazBNVGMwTDBRME9UazBMbXB3Wnd8MzUxYTFkOTQ0M2I2ODk2MzQxNWY5ZjUxNmI1NDlhYTNkZmZmZmVlYzBlYWE1NjdmNjdlYjUyY2MxOWE2Mzk5Zg)
* 点击“查询”可查看预计发货日期,仅供参考。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
| 产品编码 | D4994 |
| 纯度/分析方法 | >98.0%(HPLC) |
| 分子式/分子量 | C__3__6H__3__0NO__2P = 539.61 |
| 外观与形状(20°C) | 固体 |
| 储存温度 | 室温 (15°C以下阴凉干燥处) |
| 储存在惰性气体下 | 存放于惰性气体之中 |
| 应避免的情况 | 空气 |
| 包装和容器 | 1G-带有塑料内管的玻璃瓶 (查看图片), 200MG-带有塑料内管的玻璃瓶 (查看图片) |
| CAS RN | 415918-91-1 |
| PubChem物质ID | 354334571 |
| MDL编号 | MFCD08561138 |
技术规格
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Optical purity(LC) | min. 98.0 ee% |
| Elemental analysis(Nitrogen) | 2.20 to 3.00 % |
物性(参考值)
GHS
相关法规
| 新化学物质备案回执号 | B1A232215481 |
运输信息
| 监管条件代码(*) |
应用
Iridium / Axially Chiral Phosphoramidite Complex Catalyzed Allylic Substitution of Allyl Carbonate Derivatives
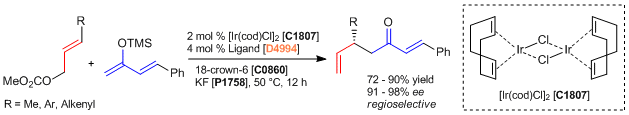
In a nitrogen-filled atmosphere, [Ir(cod)Cl]2 (2.7 mg, 0.0040 mmol), the ligand (4.3 mg, 0.0080 mmol), KF (12 mg, 0.20 mmol), 18-crown-6 (53 mg, 0.20 mmol) and a Teflon-coated magnetic stirring bar are added to a 1-dram vial. Then, anhydrous THF (0.40 mL) is added. The mixture is stirred for 5 min. at ambient temperature before allyl carbonate derivative (0.20 mmol) and the silyl enolate derivative (0.40 mmol, 2.0 equiv) are added. The vial is sealed with a cap and stirred at 50 °C for 12 h. Then, water (1 mL) is added to the reaction mixture followed by 1 N HCl (0.5 mL). The resulting mixture is stirred vigorously for 3 h at room temperature. Brine (1 mL) and Et2O (1 mL) are added; the organic layer is separated, and the aqueous layer is extracted with Et2O (3 x 3 mL). The combined organic extracts are dried over anhydrous magnesium sulfate, filtered, and concentrated under reduced pressure. Purification of the crude product is performed by flash column chromatography (gradient elution: hexane:Et2O = 50:1 to 5:1) to provide the product.
References
- Iridium-Catalyzed Enantioselective Allylic Substitution of Unstabilized Enolates Derived from α,β-Unsaturated Ketones
参考文献



![(R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine (R,R,R)-(3,5-Dioxa-4-phosphacyclohepta[2,1-a:3,4-a']dinaphthalen-4-yl)bis(1-phenylethyl)amine](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)


