Maximum quantity allowed is 999
CAS RN: 19350-66-4 | 产品编码: B6316
3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid

纯度/分析方法: >95.0%(T)(HPLC)
- 3,5-双(乙氧羰基)-2,6-二甲基-1,4-二氢吡啶-4-甲酸
- 2,6-二甲基-1,4-二氢-吡啶-3,4,5-三甲酸3,5-二乙酯
- 3,5-双(乙氧羰基)-2,6-二甲基-1,4-二羟基-4-吡啶甲酸
- 2,6-Dimethyl-1,4-dihydro-pyridine-3,4,5-tricarboxylic Acid 3,5-Diethyl Ester
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydro-4-pyridinecarboxylic Acid
* 无具体发货日期的情况,如:显示“8个工作日后发货”,将在您订购日起的8个工作日后发货。
* 我们将以最优方式从上海/天津两大仓库发货。国内库存不足,需两周左右向日本总部调货。
* 对于可分装产品,11:30前的订单,当天发货;11:30后的订单,隔天发货。
* 如需大包装,请点击“大包装询价”按钮(对于某些产品我们无法提供大包装)。
* TCI会经常复审储藏条件以对其进行优化,请以在线目录为准,敬请留意。
* 更多信息,请联系营业部:021-67121386 / Sales-CN@TCIchemicals.com 。任何货期、规格或包装方面的需求,请联系我们 。
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| 熔点 | 220 °C |
| 象形图 |

|
| 信号词 | 警告 |
| 危险性说明 | H315 : 造成皮肤刺激。 H319 : 造成严重眼刺激。 |
| 防范说明 | P264 : 作业后彻底清洗皮肤。 P280 : 戴防护手套/戴防护眼罩/戴防护面具。 P302 + P352 : 如皮肤沾染:用水充分清洗。 P337 + P313 : 如仍觉眼刺激:求医/就诊。 P305 + P351 + P338 : 如进入眼睛:用水小心冲洗几分钟。如戴隐形眼镜并可方便地取出,取出隐形眼镜。继续冲洗。 P362+P364 : 脱掉沾污的衣服,清洗后方可重新使用。 P332 + P313 : 如发生皮肤刺激:求医/就诊。 |
| 新化学物质备案回执号 | B1A232230294 |
| 监管条件代码(*) |
-
Used Chemicals
-
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid [B6316]
- 2,3,5-Tri-O-benzyl-α/β-D-ribofuranose (1)
- 4-Dimethylaminopyridine (= DMAP) [D1450]
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (= EDCI) [D1601]
- Dichloromethane
- Sodium Carbonate [S0560]
- 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (= 4CzIPN)
- Methyl 4-Bromobenzoate [B0556]
- 2,2'-Bipyridyl (= bpy) [B0468]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (= NiBr2・DME) [N1050]
- 1,4-Dioxane
-
Procedure
-
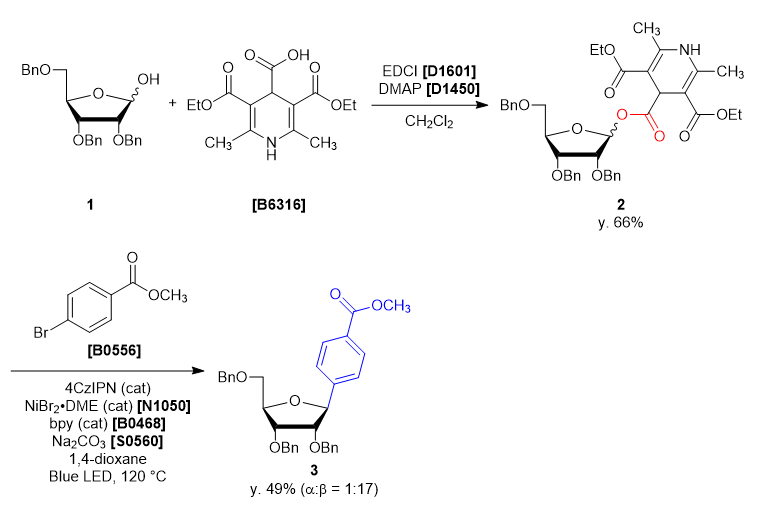
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%). -
-
Experimenter’s Comments
-
- NiBr2・DME was weighed in a nitrogen-filled glove box and dissolved in 1,4-dioxane completely using a sonication.
- 1,4-Dioxane was degassed with nitrogen for 30 min before use.
- Irradiation of visible light was performed with Kessil A160WE Tuna Blue 40W x 2.
- The reaction mixture was monitored by 1H NMR and UPLC.
- The α/β selectivity of 3 was 1:17.
-
Analytical Data
-
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
-
Lead Reference
-
- Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis
-
Other Reference
-
- Highly stereoselective synthesis of aryl/heteroaryl-C-nucleosides via the merger of photoredox and nickel catalysis
文章/手册
化学品安全说明书(SDS)
如需更多帮助,请联系我 们。
技术规格
CoA及其他文档
示例 CoA
目前没有该产品的 CoA 示例。
分析图谱
很抱歉,您搜索的分析图谱无法提供。






