Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Merci de sélectionner la quantité
New Type of Oxidation-Reduction Condensation
No.143(August 2010)

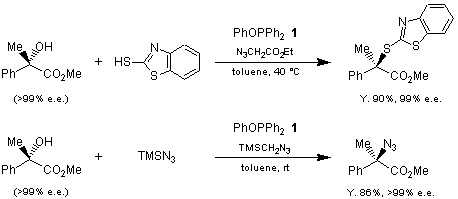
Thioetherification and azidation of tertiary alcohols by a new type of oxidation-reduction condensation using phenoxydiphenylphosphine (= phenyl diphenylphosphinite, 1) and azide compounds are reported by Mukaiyama and Kuroda’s group. These reactions proceed under mild and neutral conditions. Chiral secondary and the tertiary alcohols having an α-ester group are converted into the corresponding chiral sulfides and azides with inversion of the configuration.

When DEAD is employed as an oxidizing agent, condensation of tertiary alcohols and 2-nitrobenzoic acid affords the corresponding ester with inversion of the configuration.
These new types of oxidation - reduction condensations using 1 are applicable to sterically-hindered tertiary alcohols whereas the Mitsunobu reaction (PPh3-DEAD) is not.
References
- 1)A New type of oxidation-reduction condensation using phenyl diphenylphosphinite
- a)K. Kuroda, Y. Maruyama, Y. Hayashi, T. Mukaiyama, Chem. Lett. 2008, 37, 836.

- b)K. Kuroda, Y. Maruyama, Y. Hayashi, T. Mukaiyama, Bull. Chem. Soc. Jpn. 2009, 82, 381.

- c)T. Mukaiyama, K. Kuroda, Y. Maruyama, Y. Hayashi, Chem. Lett. 2008, 37, 1072.

- d)Review: T. Mukaiyama, K. Kuroda, Y. Maruyama,Heterocycles 2010, 80, 63.

- a)K. Kuroda, Y. Maruyama, Y. Hayashi, T. Mukaiyama, Chem. Lett. 2008, 37, 836.
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

