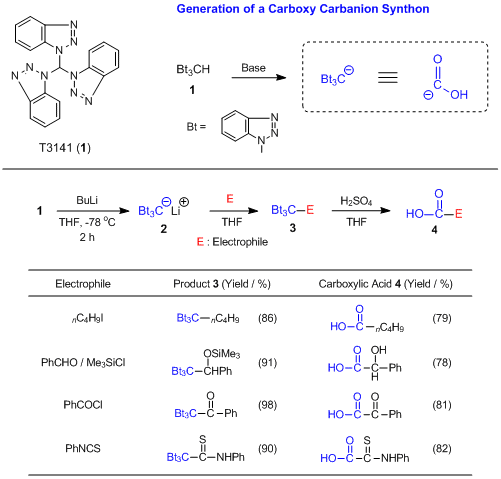
Tris(1H-benzotriazol-1-yl)methane (
1) is a three benzotriazolyl group-substituted methane derivative and it can be used as a precursor of a carboxy carbanion synthon.
1) The active methine of
1 is easily deprotonated by the action of butyl lithium to form a lithiated intermediate
2. Consequent reactions with various electrophiles produce the adducts
3. The tri-benzotriazolyl moiety of the given products
3 are converted to the corresponding carboxylic acid moiety by an acid-catalyzed hydrolysis. Therefore, the lithiated intermediate
2 is considered to be a carboxy carbanion synthon. Other carboxy carbanion synthons such as cyanide ion or lithiated orthothioesters
2) have disadvantages like toxicity and a sulfur odor. Thus,
1 is a useful precursor of a carboxy carbanion synthon because it is easy to handle.