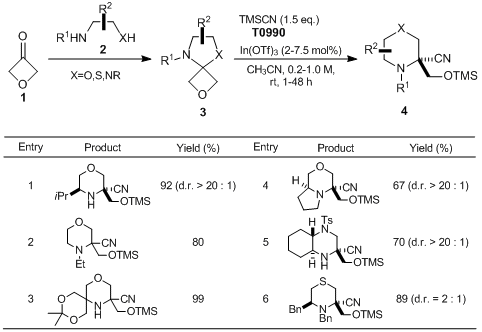
Carreira
et al. have reported the synthesis of saturated nitrogen heterocycles via ring expansion of 3-oxetanone-derived spirocycles. According to their results, 3-oxetanone readily undergoes condensation with β-heteroatom-substituted amino compounds to form related spirocycles
3. Subsequent ring expansion of
3 proceeds by the treatment with In(OTf)
3 as a Lewis acid in the presence of
TMSCN, affording the corresponding saturated nitrogen heterocycles
4 with excellent diastereoselectivity. This reaction is broad in scope and provides convenient access to valuable morpholines, thiomorpholines, and piperazines relevant to drug discovery.