Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 358-23-6 | Numéro de produit: T1100
Trifluoromethanesulfonic Anhydride

Pureté: >98.0%(T)
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 10G |
48,00 €
|
≥40 | Contactez-nous |
|
| 25G |
95,00 €
|
11 | ≥100 |
|
| 250G |
522,00 €
|
2 | ≥100 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | T1100 |
| Pureté / Méthode d'analyse | >98.0%(T) |
| Formule moléculaire / poids moléculaire | C__2F__6O__5S__2 = 282.13 |
| Etat physique (20 ° C) | Liquid |
| Condition de stockage | Refrigerated (0-10°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Moisture Sensitive,Heat Sensitive |
| CAS RN | 358-23-6 |
| Numéro de registre de Reaxys | 1813600 |
| Identifiant de la substance PubChem | 87576894 |
| SDBS | 13774 |
| Numéro MDL | MFCD00000408 |
| Appearance | Colorless to Almost colorless clear liquid |
| Purity(Neutralization titration) | min. 98.0 % |
| NMR | confirm to structure |
| Point de fusion | -80 °C |
| Point d'ébullition | 84 °C |
| Densité (20/20) | 1.72 |
| Indice de réfraction | 1.32 |
| Pictogramme |

|
| Mot de signal | Danger |
| Mentions de danger | H314 : Provoque des brûlures de la peau et de graves lésions des yeux. H290 : Peut être corrosif pour les métaux. |
| Conseils de prudence | P280 : Porter des gants de protection/ des vêtements de protection/ un équipment de protection des yeux/ du visage/ auditive. P390 : Absorber toute substance répandue pour éviter qu'elle attaque les matériaux environnants. P303 + P361 + P353 : EN CAS DE CONTACT AVEC LA PEAU (ou les cheveux): Enlever immédiatement tous les vêtements contaminés. Rincer la peau à l'eau. P301 + P330 + P331 : EN CAS D'INGESTION: Rincer la bouche. NE PAS faire vomir. P304 + P340 + P310 : EN CAS D’INHALATION: transporter la personne à l’extérieur et la maintenir dans une position où elle peut confortablement respirer. Appeler immédiatement un CENTRE ANTIPOISON/un médecin. P305 + P351 + P338 + P310 : EN CAS DE CONTACT AVEC LES YEUX: Rincer avec précaution à l'eau pendant plusieurs minutes. Enlever les lentilles de contact si la victime en porte et si elles peuvent être facilement enlevées. Continuer à rincer. Appeler immédiatement un CENTRE ANTIPOISON/un médecin. |
| Numéros CE | 206-616-8 |
| Numéro UN | UN3265 |
| Classe | 8 |
| Groupe d'emballage (DOT-AIR) | II |
| N ° SH (import / export) (TCI-E) | 2904100090 |

-
Used Chemicals
-
Procedure
-
Under nitrogen atmosphere, triphenylphosphine oxide (1.057 g, 3.798 mmol) was dissolved in dichloromethane (20 mL) and cooled to 0 °C. Then trifluoromethanesulfonic anhydride anhydride (0.295 mL, 1.80 mmol) was added and stirred for 1 h. After confirming the formation of intermediate 1 by 31P-NMR, PPTS (0.452 g, 1.80 mmol) was added and the mixture was stirred for 30 min. After that, a solution of triethylamine (0.28 mL, 2.00 mmol) and pentafluorophenol (0.370 g, 2.00 mmol) in dichloromethane (5 mL) was added dropwise with a syringe to this solution. Then the mixture was warmed to room temperature and stirred for 1 h. After completion of the reaction, the mixture was diluted with dichloromethane (20 mL) and then 2 mol/L HCl (30 mL) was added to quench. The two layers were separated and the organic layer was washed with brine (20 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel chromatography (hexane:ethyl acetate = 9:1) to afford pentafluorophenyl p-toluenesulfonate as a yellow solid (540 mg, 88% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by 1H-NMR and 31P-NMR (CDCl3).
Dichloromethane was used for the reaction after dehydration with molecular sieves and bubbling with nitrogen.
-
Analytical Data
-
Pentafluorophenyl p-Toluenesulfonate
1H NMR (400 MHz, CDCl3); δ 7.86 (d, J = 8.4 Hz, 2H), 7.42 (d, J = 8.2 Hz, 2H), 2.51(s, 3H).
-
Lead Reference
-
- Direct Synthesis of Sulfonamides and Activated Sulfonate esters From Sulfonic Acids
-
Other Reference
-
- The Structure of Polymer-Supported Triphenylphosphine Ditriflate: A Potentially Useful Reagent in Organic Synthesis

-
Used Chemicals
-
Procedure
-
Tf2O (0.32 mL, 2.0 mmol, 1.3 eq.) was slowly added to a solution of 4-bromobenzamide (300 mg, 1.5 mmol), and triethylamine (0.54 mL, 3.9 mmol, 2.6 eq.) in dichloromethane (3 mL) at 0 °C. The mixture was stirred at room temperature for 2 hours. Then additional triethylamine (1.1 mL, 7.8 mmol, 5.2 eq.) and Tf2O (0.64 mL, 3.9 mmol, 2.6 eq.) were added at 0 °C and the mixture was stirred at r.t. for overnight. The reaction mixture was quenched with water (15 mL) and the aqueous layer was extracted with ethyl acetate (15 mL x 3). The combined organic layer was washed with 2 mol/L HCl aq. (15 mL), sat. NaHCO3 aq. (15 mL), and brine (15 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by column chromatography (on silica gel, ethyl acetate:hexane = 1:8) to give 4-bromobenzonitrile a pale yellow solid (165 mg, 61% yield).
-
Experimenter’s Comments
-
The reaction mixture was monitored by UPLC.
-
Analytical Data
-
4-bromobenzonitrile
1H NMR (400 MHz, CDCl3); δ 7.64 (d, J = 8.4 Hz, 2H), 7.53 (d, J = 8.4 Hz, 2H).
-
Lead Reference
-
- A Practical Method for the Preparation of Nitriles from Primary Amides Under Non-Acidic Conditions
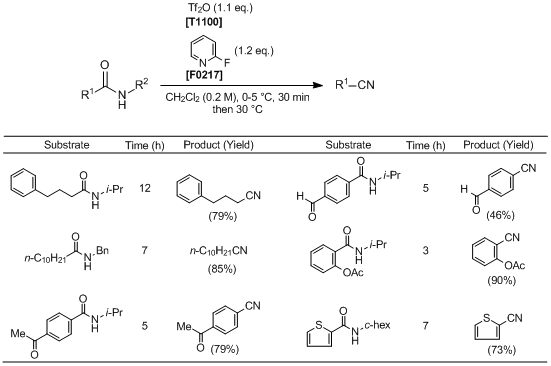
To an ice bath-cooled solution of a secondary amide (1.0 mmol) and 2-fluoropyridine (1.2 mmol) in anhydrous CH2Cl2 (5 mL) is added Tf2O (1.1 mmol). After being stirred for 0.5 h, the reaction mixture is warmed to 30 °C and stirred until completion of the reaction (monitored by TLC analysis). The reaction is quenched with 1 M HCl (1.0 mL), and the mixture is extracted with CH2Cl2 (3×10 mL). The combined organic layers are washed with saturated sodium carbonate (5 mL) and brine (5 mL), dried over anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The residue is purified by column chromatography on silica gel (ethyl acetate/petroleum ether) to afford the corresponding nitrile.
References
References
- J. E. McMurry, W. J. Scott, Tetrahedron Lett. 1980, 21, 4313.

- H. J. Bestmann, K. Kumar, W. Schaper, Angew. Chem. Int. Ed. Engl. 1983, 22, 167.

- P. J. Stang, M. Hanack, L. R. Subramanian, Synthesis 1982, 85.

Articles / Brochures
[TCI Practical Example] Dehydration of a Carbamoyl Group Using Trifluoromethanesulfonic Anhydride
Fiche de sécurité (FDS)
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Exemple de CoA
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.





