Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 19350-66-4 | Numéro de produit: B6316
3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid

Pureté: >95.0%(T)(HPLC)
- 2,6-Dimethyl-1,4-dihydro-pyridine-3,4,5-tricarboxylic Acid 3,5-Diethyl Ester
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydro-4-pyridinecarboxylic Acid
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 1G |
€86.00
|
Contactez-nous | 14 |
|
| 10G |
€264.00
|
1 | 8 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | B6316 |
| Pureté / Méthode d'analyse | >95.0%(T)(HPLC) |
| Formule moléculaire / poids moléculaire | C__1__4H__1__9NO__6 = 297.31 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Air Sensitive |
| CAS RN | 19350-66-4 |
| Numéro de registre de Reaxys | 489397 |
| Identifiant de la substance PubChem | 468591034 |
| Numéro MDL | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| Point de fusion | 220 °C |
| Pictogramme |

|
| Mot de signal | Attention |
| Mentions de danger | H315 : Provoque une irritation cutanée. H319 : Provoque une sévère irritation des yeux. |
| Conseils de prudence | P264 : Se laver la peau soigneusement après manipulation. P280 : Porter des gants de protection/ un équipement de protection des yeux/ du visage. P302 + P352 : EN CAS DE CONTACT AVEC LA PEAU: Laver abondamment à l’eau. P337 + P313 : Si l'irritation oculaire persiste: consulter un médecin. P362 + P364 : Enlever les vêtements contaminés et les laver avant réutilisation. P332 + P313 : En cas d'irritation cutanée: consulter un médecin. |
| N ° SH (import / export) (TCI-E) | 2933399990 |
-
Used Chemicals
-
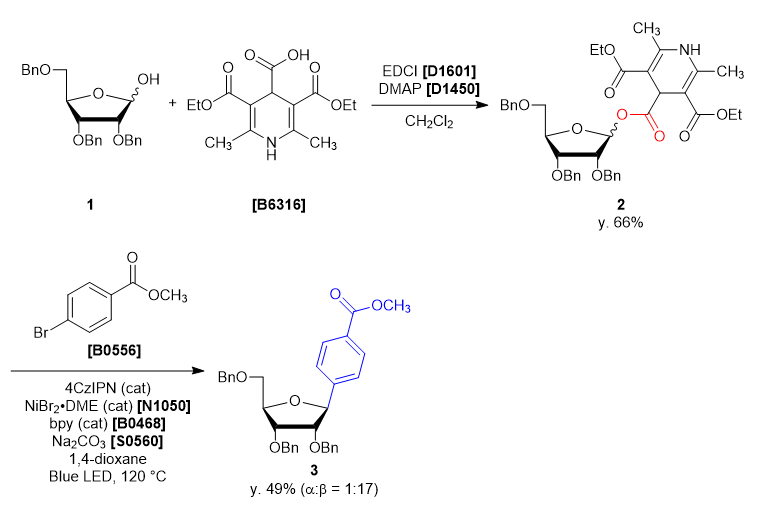
- 3,5-Bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic Acid [B6316]
- 2,3,5-Tri-O-benzyl-α/β-D-ribofuranose (1)
- 4-Dimethylaminopyridine (= DMAP) [D1450]
- 1-(3-Dimethylaminopropyl)-3-ethylcarbodiimide Hydrochloride (= EDCI) [D1601]
- Dichloromethane
- Sodium Carbonate [S0560]
- 1,2,3,5-Tetrakis(carbazol-9-yl)-4,6-dicyanobenzene (= 4CzIPN)
- Methyl 4-Bromobenzoate [B0556]
- 2,2'-Bipyridyl (= bpy) [B0468]
- Nickel(II) Bromide Ethylene Glycol Dimethyl Ether Complex (= NiBr2・DME) [N1050]
- 1,4-Dioxane
-
Procedure
-
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%). -
-
Experimenter’s Comments
-
- NiBr2・DME was weighed in a nitrogen-filled glove box and dissolved in 1,4-dioxane completely using a sonication.
- 1,4-Dioxane was degassed with nitrogen for 30 min before use.
- Irradiation of visible light was performed with Kessil A160WE Tuna Blue 40W x 2.
- The reaction mixture was monitored by 1H NMR and UPLC.
- The α/β selectivity of 3 was 1:17.
-
Analytical Data
-
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
-
Lead Reference
-
- Diastereoselective Synthesis of Aryl C-Glycosides from Glycosyl Esters via C-O Bond Homolysis
-
Other Reference
-
- Highly stereoselective synthesis of aryl/heteroaryl-C-nucleosides via the merger of photoredox and nickel catalysis
Articles / Brochures
Fiche de sécurité (FDS)
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Exemple de CoA
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.





