Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
CAS RN: 32005-36-0 | Numéro de produit: B1374
Bis(dibenzylideneacetone)palladium(0)

Pureté:
- Palladium(0) Bis(dibenzylideneacetone)
- Bis(dba)palladium(0)
- Pd(dba)2
| Taille | Prix unitaire | Belgique | Japon * | Quantité |
|---|---|---|---|---|
| 1G |
€ 116,00
|
14 | ≥100 |
|
| 5G |
€ 465,00
|
6 | ≥100 |
|
*Le délai de livraison pour des produits disponibles en stock en Belgique est 1 à 2 jours
*Le délai de livraison pour des produits disponibles en stock en Japon est 1 à 2 semaines (sauf des produits réglementés et des envois avec de la glace carbonique)
| Numéro de produit | B1374 |
| Formule moléculaire / poids moléculaire | C__3__4H__2__8O__2Pd = 575.02 |
| Etat physique (20 ° C) | Solid |
| Condition de stockage | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Stocker sous gaz inerte | Store under inert gas |
| Condition à éviter | Air Sensitive |
| Emballage Et Conteneur | 1G-Glass Bottle with Plastic Insert (Voir l'image) |
| CAS RN | 32005-36-0 |
| Identifiant de la substance PubChem | 87564299 |
| SDBS | 50493 |
| Numéro MDL | MFCD00051942 |
| Appearance | Brown to Black powder to crystal |
| Content (Palladium) | 16.5 to 20.5 % |
| Point de fusion | 150 °C |
| Pictogramme |

|
| Mot de signal | Attention |
| Mentions de danger | H302 + H312 + H332 : Nocif en cas d’ingestion, de contact cutané ou d’inhalation. H315 : Provoque une irritation cutanée. H319 : Provoque une sévère irritation des yeux. |
| Conseils de prudence | P261 : Éviter de respirer les poussières. P264 : Se laver la peau soigneusement après manipulation. P280 : Porter des gants de protection/ des vêtements de protection/ un équipment de protection des yeux/ du visage/ auditive. P337 + P313 : Si l'irritation oculaire persiste: consulter un médecin. P302 + P352 + P312 : EN CAS DE CONTACT AVEC LA PEAU: Laver abondamment à l’eau. Appeler un CENTRE ANTIPOISON/un médecin en cas de malaise. P304 + P340 + P312 : EN CAS D’INHALATION: transporter la personne à l’extérieur et la maintenir dans une position où elle peut confortablement respirer. Appeler un CENTRE ANTIPOISON/un médecin en cas de malaise. |
| N ° SH (import / export) (TCI-E) | 2843909000 |
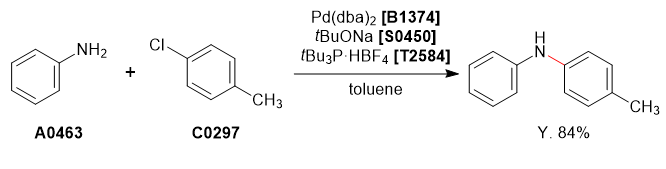
Used Chemicals
Procedure
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Experimenter’s Comments
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
Analytical Data (4-Methyl-N-phenylaniline)
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).
Lead Reference
- Tetramethoxybenzene is a Good Building Block for Molecular Wires: Insights from Photoinduced Electron Transfer
Used Chemicals
- 2-Chloro-m-xylene [C2164]
- 2-Methylphenylboronic Acid [M1313]
- Bis(dibenzylideneacetone)palladium (0) (Pd(dba)2) [B1374]
- 2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) [D5036]
- Tripotassium Phosphate (K3PO4)
- Toluene
- Ion-exchange water
Procedure
To a 3-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (66 mg, 0.115 mmol, 1.5 mol%), SPhos (94 mg, 0.229 mmol, 3.0 mol%), 2-methylphenylboronic acid (1.56 g, 11.5 mmol, 1.5 equiv.), tripotassium phosphate (4.87 g, 22.9 mmol, 3.0 equiv.) degassed toluene (15 mL), and ion-exchange water (1.5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 2-Chloro-m-xylene (1.0 mL, 7.64 mmol, 1.0 equiv.) was added one portion. The resulting mixture was stirred at reflux for 7 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane only) to afford the corresponding compound as a colorless oil (1.46 g, 97%).
Experimenter's Comments
i) Toluene was degassed by bubbling with nitrogen gas for 30 min.
ii) The reaction mixture was monitored by GC.Analytical Data(2,2',6-Trimethyl-1,1'-biphenyl)
1H NMR (400 MHz, CDCl3); δ 7.23-7.33 (m, 3H), 7.22-7.10 (m, 3H), 7.00-7.06 (m, 1H), 1.99 (s, 3H), 1.97 (s, 6H).
13C NMR (101 MHz, CDCl3); δ 141.0, 140.5, 135.8, 135.5, 129.9, 128.8, 127.2 127.0, 126.9, 126.0, 20.3, 19.4.
Lead Reference
- Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure
Used Chemicals
Procedure
-
To a 2-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (36 mg, 0.0633 mmol, 1.5 mol%), XPhos (60 mg, 0.127 mmol, 3.0 mol%), sodium tert-butoxide (811 mg, 8.44 mmol, 2.0 equiv.) and toluene (5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 4-chlorotoluene (0.5 mL, 4.22 mmol, 1.0 equiv.), and morpholine (0.55 mL, 6.33 mmol, 1.5 equiv.) were added in one portion. The resulting mixture was stirred at reflux for 6 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane : ethyl acetate = 9 : 1) to afford the corresponding compound as an orange solid (700 mg, 94%).
Experimenter's Comments
Toluene was degassed by bubbling with nitrogen gas for 30 min.
The reaction mixture was monitored by GC.Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.10 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 3.87 (t, J = 4.8 Hz, 4H), 3.11 (t, J = 4.8 Hz, 4H), 2.28 (s, 3H).
13C NMR (101 MHz, CDCl3); δ 149.1, 129.7, 129.5, 116.0, 66.9, 49.9, 20.4.
Lead Reference
- Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions
- Pd-Catalyzed Kumada-Corriu Cross-Coupling Reactions at Low Temperatures Allow the Use of Knochel-type Grignard Reagents


References
Articles / Brochures
Fiche de sécurité (FDS)
La FDS demandée n'est pas disponible.
Nous contacter pour plus d'informations.
Spécifications
CoA et autres Certificats
Exemple de CoA
Un échantillon CoA pour ce produit n'est pas disponible pour le moment.
Graphiques analytiques
Le tableau analytique demandé n'est pas disponible. Nous sommes désolés pour ce désagrément.







![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)

