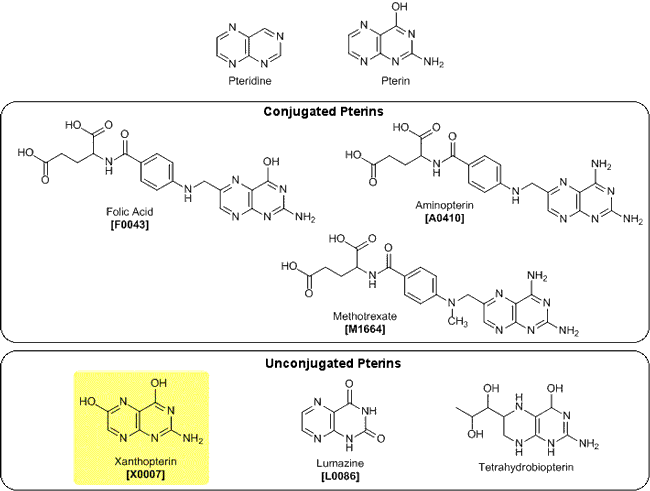
The term pteridine is generally used to refer to hetero-bicyclic compounds containing four nitrogen atoms. Specifically, the structure of 2-amino-4-hydroxypteridine is known as pterin, whose derivatives are found in many kinds of organisms.1) Pterins substituted by long side chains at the 6-position are classified as "conjugated" pterins and include folic acid, famous for vitamin B9 and the folate metabolism antagonists: aminopterin and methotrexate. On the other hand, in "unconjugated" pterins substituted by short side chains, there are lumazine, a basic skeleton of dye blue-shifting bacterial luciferase luminescence";" tetrahydrobiopterin, an essential cofactor of the aromatic amino acid hydroxylases, etc.
Xanthopterin is an "unconjugated" pterin isolated as the yellow dye of butterfly wings by H. Wieland et al. in 1925,2) and the structure determination was performed in 1940.3) It has been reported that xanthopterin also appears in mammalian urine as a metabolite of tetrahydrobiopterin derived from guanosine triphosphate.4,5)
Surprisingly, the oriental hornet (Vespa orientalis) makes the most of the natural dye xanthopterin. In M. Plotkin's report, they may harvest solar energy in the yellow-colored cuticle containing xanthopterin with solar insolation.6)