Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
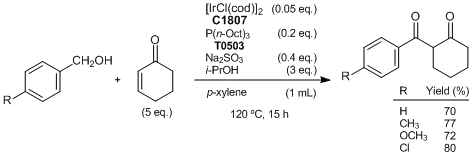
Iridium-catalyzed reaction of alcohols with enones affording 1,3-diketones
Obora et al. have reported a [IrCl(cod)]2 catalyzed selective coupling reaction of alcohols with enones to give 1.3-diketones, which are widely used as building blocks for heterocyclic synthesis. They have suggested the reaction pathway as follows. The reaction is initiated by formation of the iridium hydride and an aldehyde via hydrogen transfer from the alcohol to [IrCl(cod)]2. Then an enone is inserted by the Ir-H bond of the iridium hydride to form hydridooxa(π-allyl)iridium as a key intermediate. Subsequently, the intermediate reacts with the aldehyde to give 1,3-diketones.