Published TCIMAIL newest issue No.196
TCI uses cookies to personalize and improve your user experience. By continuing on our website, you accept the use of cookies. You can change or update your cookiesettings at any time.
Maximum quantity allowed is 999
Please select the quantity
Molecular Conductors
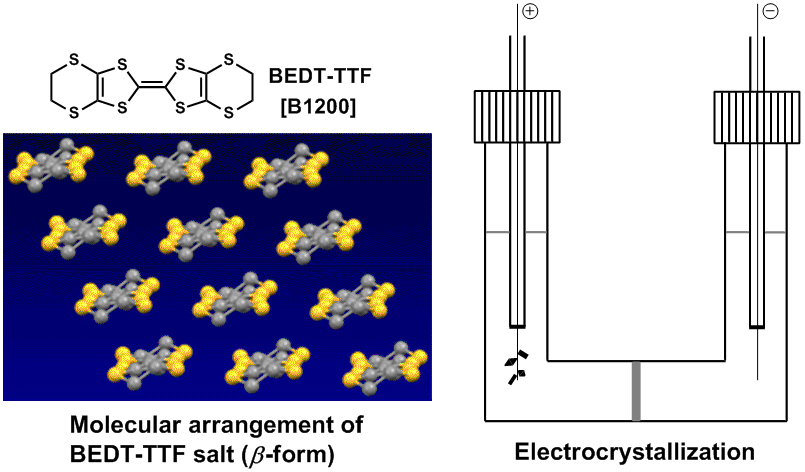
A molecular conductor is an electrical conductor based on a molecular component. A chemical modification of the molecule can control the electronic structure and physical properties. We can synthesize an opened-shell molecular conductor by chemical or electrochemical doping of a carrier, although an organic molecule is usually an insulator with a closed-shell structure. The first example of an organic conductor was observed from a bromine-doped perylene.1) After this observation, a molecular conductor based on tetrathiafulvalene (TTF) was reported in the 1970s,2) and the first case of an organic molecular superconductor was observed from the organic salt of tetraselenafulvalene, (TMTSF)2X in the 1980s.3) These TTF and TMTSF salts form a one-dimensional or pseudo one-dimensional molecular arrangement. On the other hand, bis(ethylenedithio)tetrathiafulvalene (BEDT-TTF) favors forming a two-dimensional molecular arrangement, which is a relatively stable molecular metal toward temperature.4-6)
Conducting salts of TTF derivatives can be normally obtained by an electrochemical oxidation (electrocrystallization).7) These TTF derivatives can function as donor molecules with a hole carrier. Metal dithiolene complexes (M(dmit)2), 7,7,8,8-Tetracyanoquinodimethane (TCNQ) and fullerene (C60) are acceptor molecules with an electron carrier. An M(dmit)2 salt produced the first example of an acceptor-based organic superconductor.8) Chemical modifications of the M(dmit)2 salt produced plenty of those organic superconductors by changing the central metal atom and counter cation.9)
Several alkali-doped nanocarbon and nanographene compounds have shown superconductivity. It has well known that rubidium- and cesium-doped fullerenes have demonstrated superconductivity at more than 30 K.10) Recently, superconductivity of Cs3C60 at 38 K was reported.11) Although the molecular conductors synthesized from TTF and M(dmit)2 exhibit low-dimensional molecular arrangements, these fullerene salts can form three-dimensionality.12) Kubozono et al. reported that an alkali-doped picene demonstrated superconductivity at 18 K.13) This result indicates that one can observe superconductivity from a planar nanocarbon material as well. In addition to the picene-based superconductor, an alkali-doped phenanthrene (Tc = 5 K),14) alkali-doped coronene (Tc = 15 K),15) and alkali-doped 1,2:8,9-dibenzopentacene (Tc = 33 K)16) have also shown superconductivity.

References
- 1) H. Akamatu, H. Inokuchi, Y. Matsunaga, Nature 1954, 173, 168.

- 2) F. Wudl, D. Wobschall, E. J. Hufnagel, J. Am. Chem. Soc. 1972, 94, 670.

- 3) D. Jérome, A. Mazaud, M. Ribault, K. Bechgaard, J. Phys. Lett. 1980, 41, 95.

- 4) T. Mori, Chem. Rev. 2004, 104, 4947.

- 5) R. P. Shibaeva, E. B. Yagubskii, Chem. Rev. 2004, 104, 5347.

- 6) H. Mori, Int. J. Mod. Phys. B 1994, 8, 1.

- 7) P. Batail, K. Boubekeur, M. Fourmigué, J.-C. P. Gabriel, Chem. Mater. 1998, 10, 3005.

- 8) L. Brossard, M. Ribault, M. Bousseau, L. Valade, P. Cassoux, C. R. Acad. Sci., Ser. Ⅱ 1986, 302, 205.
- 9) R. Kato, Chem. Rev. 2004, 104, 5319.

- 10) K. Tanigaki, T. W. Ebbesen, S. Saito, J. Mizuki, J. S. Tsai, Y. Kubo, S. Kuroshima, Nature 1991, 352, 222.

- 11) A. Y. Ganin, Y. Takabayashi, Y. Z. Khimyak, S. Margadonna, A. Tamai, M. J. Rosseinsky, K. Prassides, Nat. Mater. 2008, 7, 367.

- 12) Y. Takabayashi, A. Y. Ganin, P. Jeglič, D. Arčon, T. Takano, Y. Iwasa, Y. Ohishi, M. Takata, N. Takeshita, K. Prassides, M. J. Rosseinsky, Science 2009, 323, 1585.

- 13) R. Mitsuhashi, Y. Suzuki, Y. Yamanari, H. Mitamura, T. Kambe, N. Ikeda, H. Okamoto, A. Fujiwara, M. Yamaji, N. Kawasaki, Y. Maniwa, Y. Kubozono, Nature 2010, 464, 76.

- 14) X. F. Wang, R. H. Liu, Z. Gui, Y. L. Xie, Y. J. Yan, J. J. Ying, X. G. Luo, X. H. Chen, Nat. Commun. 2011, 2, 1513.

- 15) Y. Kubozono, H. Mitamura, X. Lee, X. He, Y. Yamanari, Y. Takahashi, Y. Suzuki, Y. Kaji, R. Eguchi, K. Akaike, T. Kambe, H. Okamoto, A. Fujiwara, T. Kato, T. Kosugi, H. Aoki, Phys. Chem. Chem. Phys. 2011, 13, 16476.

- 16) M. Xue, T. Cao, D. Wang, Y. Wu, H. Yang, X. Dong, J. He, F. Li, G. F. Chen, Sci. Rep. 2012, 2, srep00389.


