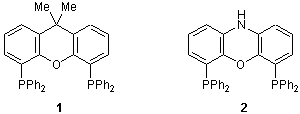
For Preparation of Large Bite Angle Metal Complexes
4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene (1), as bidentate phosphine ligand, forms stable complexes with transition metals such as palladium and rhodium. These complexes have a large bite angle, and they are used in organic synthetic reactions as highly selective catalysts.

For example, Kranenburg and co-workers carried out an alkylation reaction between 2-hexenyl acetate and the carbanin of diethyl malonate in the presence of various bidentate phosphine ligands and palladium, and made a comparative study on the reactivity of the various products derived. According to this research, the palladium complex catalyst in which 1 is used as the ligand has the largest bite angle and its selectivity is very high. Moreover, they reported that only diethyl 2-(2-hexane-1-yl)-2-methyl malonate (3) was produced, with no diethyl 2-(1-hexane-3-yl)-2-methyl malonate (4) by-product.1) In addition, Kranenburg and co-workers reported that n-aldehyde is formed with high selectivity in the hydroformylation reaction of 1-olefin in which the complex composed of 1 and rhodium is used as the catalyst.2)

On the other hand, Sandee and co-workers used 4,6-bis(diphenylphosphino)phenoxazine (2) as a starting material to synthesize the silica gel-supported rhodium complex catalyst 5. This catalyst 5 is also utilized for highly selective hydroformylation reactions since it too has a large bite angle nearly equal to that of the complex catalyst composed of 1 and rhodium, and it is easy to recover and reuse.3)
References
- The effect of the bite angle of diphosphine ligands
- 1)M. Kranenburg, P. C. J. Kamer, P. W. N. M. van Leeuwen, Eur. J. Inorg. Chem. 1998, 25.

- 2)M. Kranenburg, Y. E. M. van der Burgt, P. C. J. Kamer, P. W. N. M. van Leeuwen, K. Goubitz, J. Fraanj, Organometallics 1995, 14, 3081.

- 3)L. A. van der Veen, P. H. Keeven, G. C. Schoemaker, J. N. H. Reek, P. C. J. Kamer, P. W. N. M. van Leeuwen, M. Lutz, A. L. Spek, Organometallics 2000, 19, 872.

- 4)A. J. Sandee, J. N. H. Reek, P. C. J. Kamer, P. W. N. M. van Leeuwen, J. Am. Chem. Soc. 2001, 123, 8468.

The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.