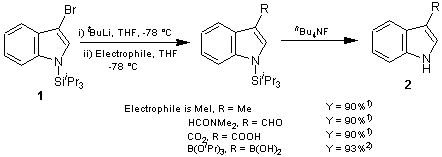
The 3-lithiated indole is generated from silylindole 1 by treatment with alkyllithium and when allowed to react at -78°C with various electrophiles, regioselectively yields 3-substituted indoles in good yield . This is because the lithiated intermediate contains a bulky triisopropylsilyl group at the 1-position. The steric bulk provides protection at the 2-position of indole which prevent 3→2 migration of lithium and subsequent ring fragmentation1).
Reported applications of 1 are the synthesis of tryptophan2) and the total synthesis of grossularine-13) which is a type of alkaloid.
Published TCIMAIL newest issue No.200