Chemistry Chat
- Focusing on the Elements - Chemistry between Carbon and Other Elements
Numerous research articles are published every day in the field of organic chemistry. Come to think of it, in the genre that deals with only one element - carbon, considering such high pace of publication, one might wonder how researchers manage not to run out of new ideas. Apparently, the possibility of carbon chemistry has a large space left to be explored after hundreds of years of history, and the horizon even continues to expand.
In the CAS database, the largest chemical database in the world, close to 70 million compounds are registered. Of those, 80 percents are carbon-based organic compounds. Within the periodic table consisting of more than 100 elements, carbon has a special status which may be called the “king of all elements.”
There are several reasons why carbon is such a special element, and one of them is because of its ability to form stable bond(s) with a number of other “heteroatom” elements. Carbon forms bonds with not only common non-metal elements such as hydrogen, oxygen, nitrogen, sulfur, phosphorus, and halogens, but also with most metallic elements. Many of the compounds based on carbon-heteroatom bond are common reagents found in the shelves of chemistry laboratories.
Without needing to mention such prime examples as Grignard reagent, hydroboration, and Wittig reaction, carbon-heteroatom bond has always been trailblazing the development of organic chemistry. Even relatively less familiar heavy metal elements such as hafnium, rhenium, and bismuth have been utilized for organic synthetic reactions. Molybdenum and ruthenium carbenes used to be somewhat exotic compounds until not too long ago, but became mainstream regulars after the advent of Grubbs olefin metathesis catalyst. The development of new chemical bond is always the most important frontier of chemistry, and as such, it continuously draws a great deal of interest and energy from scientific community.
Page Top
Organic Noble Gas Compounds
The synthesis of the first xenon-containing compound XePtF6 by Neil Bartrett in 1962 is a renowned accomplishment listed in many textbooks. Since then, xenon compounds of different oxidation numbers (II, IV, VI, VIII) have been known to date and many of those are fluorides and oxides. Among them, for example, XeF2 is a useful (and commercially available) fluorination reagent.
Xenon is also known to form a bond with carbon. It has a surprisingly long history, with the first preparation of its kind reported back in 1989 [1]. For Xe(II) compound, C6F5XeF has been used to synthesize several organic xenon compounds including Xe(C6F5)2 [2].
In 2000, the first organic xenon(IV) compound was synthesized [3]. For example, [C6F5XeF2]BF4 was made by the reaction between XeF4 and C6F5BF2. Reported to act as a strong fluorinating agent, this compound perhaps has an interesting chance to develop into a reagent.
For krypton, which is one size smaller family member of xenon, it is known that photo irradiation in the presence of acetylene under low temperature conditions produces HC≡CKrH [4]. There might be similar possibility for radon, but so far only Rn(II) fluoride is known and the synthesis of organic radon compound has not been realized. How about argon and neon? To find out whether they can react with carbon, there is probably a competition going on among laboratories around the world.
Page Top
Hot Organic Compounds
High sociability of carbon extends to the realm of radioactive elements. For example, short-lived astatine (having a half-life of maximum 8.1 hours) forms organic compounds such as C6H5At. Actinide elements such as uranium, neptunium, plutonium, and americium also complex with cyclooctatetraene to form sandwich-shaped molecules commonly called “hot sandwiches” (picture shown below) [5]. These complexes are rare molecules having a symmetry feature known as D8h. For uranium, there is Cp4U complex, in which four cyclopentadienyl ligands are bonded to the uranium in tetrahedral fashion [6]. Big atoms have surprising properties indeed.
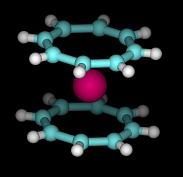
"Hot sandwiches"
To the best of my literature search, the heaviest element that forms a bond with carbon is einsteinium with atomic number 99. In 2005, the synthesis of an alkene-einsteinium complex was reported [7]. Among smaller elements than this, there are only a few which are not known to bond with carbon; the examples are some of noble gases like helium, neon, and argon, and extremely short-lived species such as francium.
By the way, if the partner is not limited to carbon, the heaviest element ever prepared as a compound is hassium with atomic number 108. In 2002, hassium compounds including HsO4 were synthesized from just seven atoms and their properties have been studied [8]. The isotope used in the study had a half life of only 11 seconds, which tells us about the incredibly high level of experimental sophistication. Considering that, there may still be rooms in the list of elements that potentially make a bond with carbon.
Page Top
The Element Not Found in The Periodic Table
In 2008, an element called muonium emerged as a new chemical partner of carbon. Most people probably have never heard of this “element”, however, because it is not found anywhere in the periodic table. Muonium is not exactly an element which consists of proton, neutron, and electron.
There is an elemental particle called muon. This particle is 207 times heavier than electron and can have either a positive or a negative charge. A positively charged muon captures an electron upon encounter to form a hydrogen-like “atom” called muonium, which is considered one of the “exotic atoms” having neither proton nor neutron.
P. W. Percival and his group used muonium as an equivalent of hydrogen radical while studying the reactivity of carbon-silicon double bonds [9]. In this study, a compound in which muonium is bonded to carbon was observed. As a research method of organic chemistry, this is a very atypical but impressive approach.
You may wonder how stable the carbon-muonium bond is. Muon itself exists with an average longevity of only 2.2 microseconds, therefore, the organic muonium species is destined to vanish in split seconds literally.
It is surprising that any meaningful data can be collected in that instantaneous period of time, but according to the experts in the field of elemental particle research, muonium actually belongs to the group of long-lived particles and its analysis is relatively easy. For example, positronium (an exotic atom composed of an electron and a positron) is also known to form a compound but it lives for nanoseconds, therefore, its detection is “slightly difficult.” The physical chemists certainly have a very different perception about time, but this kind of interdisciplinary laboratory should be a great place where fascinating researches are born.
References
- [1] D. Naumann, W. Tyrra, J. Chem. Soc. Chem. Commun. 1989, 47; H.-J. Frohn , S. Jakobs, J. Chem. Soc. Chem. Commun. 1989, 625.

- [2] H.-J. Frohn, M. Theissen, Angew. Chem. Int. Ed. 2000, 39, 4591.

- [3] H.-J. Frohn et al., Angew. Chem. Int. Ed. 2000, 39, 391.

- [4] L. Khriachtchev et al., J. Am. Chem. Soc. 2003, 125, 6876.

- [5] A. Streitwieser, U. Mueller-Westerhoff, J. Am. Chem. Soc. 1968, 90, 7364; D. G. Karraker et al., J. Am. Chem. Soc. 1970, 92, 4841.

- [6] J. H. Burns, J. Organomet. Chem. 1974, 69, 225.

- [7] J. K. Gibson, R. G. Haire, Radiochimica Acta 2003, 91, 441.

- [8] E. Düllmann et al., Nature 2002, 418, 859.
- [9] B. M. McCollum et al., Angew. Chem. Int. Ed. 2008, 47, 9772.

Page Top
Introduction of the author
Kentaro Sato
[Brief career history] He was born in Ibaraki, Japan, in 1970. 1995 M. Sc. Graduate School of Science and Engineering, Tokyo Institute of Technology. 1995-2007 Researcher in a pharmaceutical company. 2008- Present Freelance science writer. 2009-2012 Project assistant professor of the graduate school of Science, the University of Tokyo.
[Specialty] Organic chemistry
Page Top
