Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Bitte wählen Sie die Menge aus.
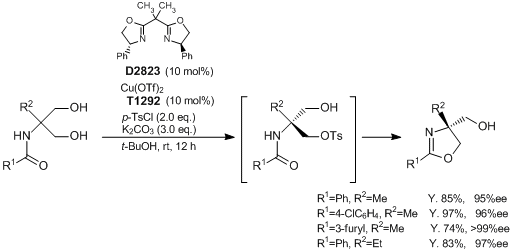
Synthesis of Optically Active Oxazoline Derivatives via Asymmetric Desymmetrization of 1,3-Diols
Onomura et al. have reported the synthesis of optically active oxazolines through a copper-catalyzed asymmetric desymmetrization of 1,3-diols. According to their results, 2-(N-acylamino)-1,3-propanediols react with p-toluenesulfonyl chloride in the presence of Cu(OTf)2 and (R,R)-2,2'-isopropylidenebis(4-phenyl-2-oxazoline) as a catalyst to give the desired optically active oxazoline derivatives having a quaternary stereocenter in good to excellent yields with high enantioselectivities. The advantages of this method are a simple and easy operation and mild reaction conditions. Since this reaction system tolerates a diverse range of substrates, it is expected to be applied to the synthesis of various biologically active agents.