Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
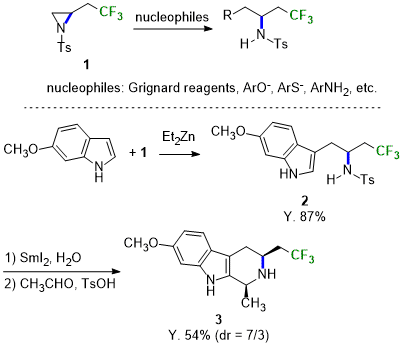
Aziridine Derivative for the Synthesis of a Trifluoroalkylamine Moiety
Sodeoka and Kawamura et al. have recently reported 1-tosyl-2-(2,2,2-trifluoroethyl)aziridine (1), which is utilized in the synthesis of trifluoroalkylamine derivatives. 1 reacts with various carbon and nitrogen nucleophiles to provide ring-opening adducts with high regioselectivity and in high yields. Furthermore, when the indole adduct 2 is treated with acetaldehyde, the tetrahydroharmine derivative containing a fluorinated functional group (3) is afforded via a Pictet-Spengler reaction. 1 is expected to be used as a building block for fluoroamine synthesis and heterocycles for pharmaceutical and agrochemical research.
Related Products
References
- N-Heterocycle-Forming Amino/Carboperfluoroalkylations of Aminoalkenes by Using Perfluoro Acid Anhydrides: Mechanistic Studies and Applications Directed Toward Perfluoroalkylated Compound Libraries

