Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
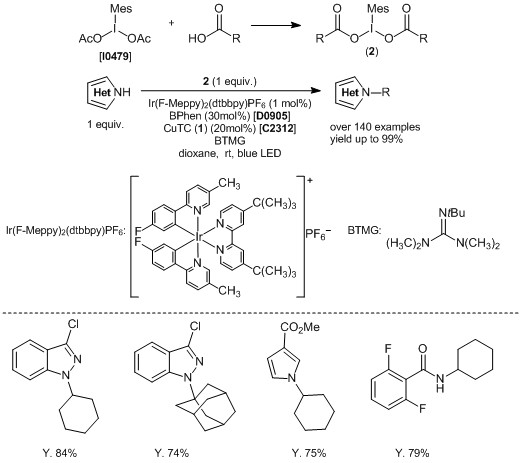
Copper/photoredox-catalyzed Decarboxylative sp3 C–N Coupling Reaction of N-Heteroaromatics
MacMillan and co-workers have recently utilized copper(I) 2-thiophenecarboxylate (1) as a catalyst for a C-N coupling reaction of N-heteroaromatic derivatives in the presence of photoredox catalyst. For example, iodomesitylene dicarboxylate (2) prepared from iodomesitylene diacetate and alkylcarboxylic acids reacts with N-heteroaromatic derivatives. The Ir complex, 1 and bathophenanthroline act as a catalyst to give the corresponding N-alkyl heteroaromatic products in good yields. This reaction can proceed when using a carboxylic acid neighboring a sterically hindered alkyl group such as adamantyl group. Furthermore, the C-N coupling is utilized by the amide nitrogen, carbonate and sulfone amides. In this way, this reaction is expected to be use in late-stage C-N coupling of research of pharmaceuticals, as well as late-stage total synthesis.
Related Products
Reference
- Decarboxylative sp3 C–N coupling via dual copper and photoredox catalysis

