Make sure to sign up for an account today for exclusive coupons and free shipping on orders over $75!
Maximum quantity allowed is 999
Please select the quantity
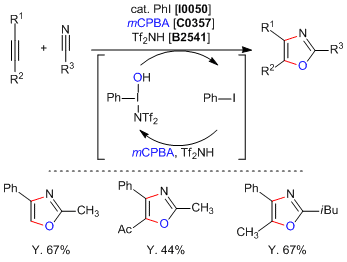
Metal-Free Construction of Oxazole Ring by Formal [2+2+1] Cycloaddition
Saito et al. have reported a metal-free synthesis of oxazole rings by a formal [2+2+1] cycloaddition. The reaction is mediated by a catalytic in situ generated iodine (III) species to cyclize an alkyne and nitrile moieties affording di or trisubistuted oxazoles with good yield and complete regioselectivity. It was experimentally determined that the hypervalent iodine moiety is formed in situ by the treatment of a catalytic amount of iodobenzene with mCPBA and bis(trifluoromethanesulfonyl)imide (= Tf2NH). This result represents the first example of a multicomponent reaction using a hypervalent iodine (III) compound.
References
- Iodine(III)-Catalyzed Formal [2 + 2 + 1] Cycloaddition Reaction for Metal-Free Construction of Oxazoles

