Sign up for free shipping on all website orders, no minimum required, and get exclusive coupons!
Maximum quantity allowed is 999
Please select the quantity
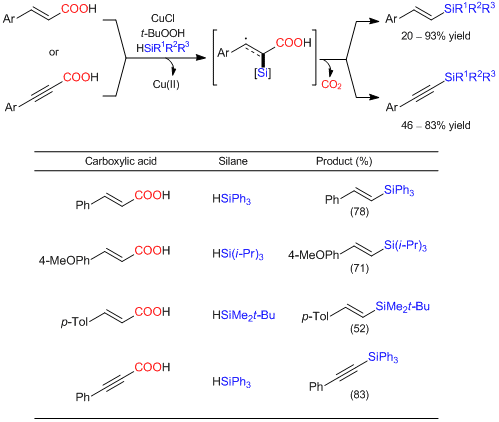
Decarboxylative silylation of α,β-unsaturated carboxylic acids
Liu et al. have reported a decarboxylative silylation of α,β-unsaturated carboxylic acids. According to their report, the decarboxylative C-Si bond-forming reaction of trans-cinnamic acid with triphenylsilane successfully proceeds in the presence of copper chloride(I) as a reaction initiator and tert-butyl hydroperoxide (TBHP). This reaction can be applied to α,β-alkenyl- and α,β-alkynyl- carboxylic acids giving the corresponding vinyl- and alkynyl- silanes. This is the first example of the decarboxylative silylation of carboxylic acids. The reaction pathway is suggested to proceed through a radical pathway initiated by SET (single electron transfer) from a copper salt.