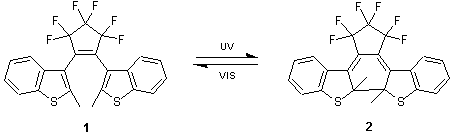
Photochromic compounds can change their molecular structures by reversible process upon irradiation of lights of two different wavelengths. Typical examples are azobenzenes, spirobenzopyrans, furylfulgides as well as diarylethenes. Diarylethenes are anticipated as highly important chemicals in the application of optical devices such as optical memories and switches since they are thermally stable, and posses repeated durability.
1,2-Bis[2-methylbenzo[b]thiophen-3-yl]-3,3,4,4,5,5-hexafluoro-1-cyclopentene (1), an example of the diarylethenes, changes to red, forming a closed structure 2 upon irradiation of UV light, but rapidly converts back to colorless, forming the open-ring structure 1 by visible light. In this reversible reaction, 1 has been shown a repeated durability over 10,000 times. Compounds 1 and 2 are known to be stable at high temperatures even up to 80 degree C,1) thus, showing a great potential as an ideal material for the optical devices. Furthermore, it may lead to the development of various new techniques in photo-mechanical effects if different functional groups are incorporated into the aromatic systems in diarylethenes.2)
Published TCIMAIL newest issue No.199