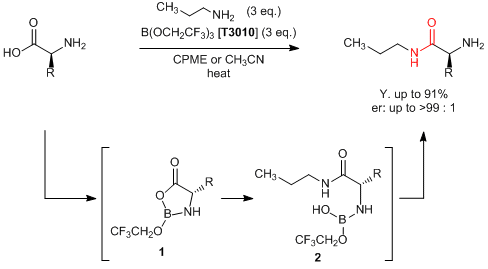
Sheppard
et al. have reported a direct amidation of unprotected amino acids with a borate ester. According to their results, unprotected amino acids react with amines by adding
tris(2,2,2-trifluoroethyl) borate to afford the corresponding amides in good yields. This reaction is proposed to proceed via the following reactions;" both amino and carboxy groups in amino acids react with the boric ester to form the cyclic intermediate
1, and affords the boric amide
2 through the nucleophilic addition of an amine to the carbonyl group of
1. Generally, condensation of amino acids requires a protection/deprotection process, which leads to multi-step reactions. On the other hand, this reaction can be directly applied to the use of unprotected amino acids and the target compounds are given in a single step. In this way, this synthetic method is expected to be applied for the development of pharmaceuticals and agrichemicals.