Published TCIMAIL newest issue No.199
Maximum quantity allowed is 999
Gelieve het aantal te selecteren
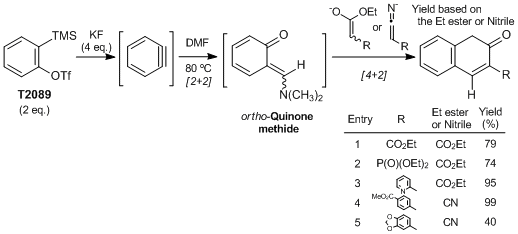
Three-Component coupling of benzyne, DMF, and active methylene compounds to produce coumarins
Yoshida et al. have reported that the novel three-component coupling using benzyne (prepared from 2-(trimethylsilyl)phenyl trifluoromethanesulfonate and KF), DMF and active methylene compounds provides direct access to coumarins. They have demonstrated that ortho-quinone methide, arising from a formal [2+2] cycloaddition between benzyne and DMF, undergoes a [4+2] cycloaddition with ester enolates or ketenimine anions to produce coumarins, which are an important class of bioactive compounds.