Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
*Voorraad beschikbaar in België: Verzending dezelfde dag
*Voorraad beschikbaar in Japan: Raadpleeg de verzendsimulatie voor geschatte levertijden. (met uitzondering van gereguleerde producten en zendingen met droogijs)
| Artikel # | T3121 |
Zuiverheid / Analysemethode 
|
>97.0%(HPLC) |
| Moleculaire formule / molecuulgewicht | C__7H__3Cl__3O__2 = 225.45 |
| Fysieke toestand (20 graden C) | Solid |
Opslag condities 
|
Room Temperature (Recommended in a cool and dark place, <15°C) |
Verpakking 
|
1G-Glass Bottle with Plastic Insert (Bekijk afbeelding) |
| CAS RN | 4525-65-9 |
| Reaxys registratienummer | 2524466 |
| PubChem product ID | 253661984 |
| Appearance | White to Almost white powder to crystal |
| Purity(HPLC) | min. 97.0 area% |
| Purity(Argentometric Titration) | min. 95.0 % |
| Melting point | 81.0 to 85.0 °C |
| Smeltpunt | 83 °C |
| Oplosbaarheid (oplosbaar in) | Toluene |
| HS-NR (invoer / uitvoer) (TCI-E) | 2915130090 |

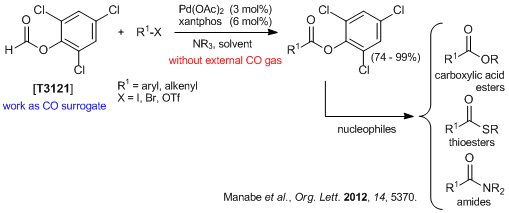
A solution of 2-iodofluorene (500 mg, 1.71 mmol), 2,4,6-trichlorophenyl formate (579 mg, 2.57 mmol), palladium(II) acetate (38.4 mg, 0.171 mmol), Xantphos (198 mg, 0.342 mmol) and triethylamine (470 µL, 3.42 mmol) in toluene (10 mL) was stirred at room temperature for 24 hours. The solvent was removed under reduced pressure and the residue was washed with acetonitrile to give 1 as a pale yellow solid (462 mg, 69% yield).
A solution of 1 (460 mg, 1.18 mmol), triethylamine (330 µL, 2.36 mmol), DMAP (7.2 mg, 59 µmol) and morpholine (154 µL, 1.77 mmol) in THF (5 mL) was stirred at 45 °C for 20 hours. The reaction was quenched with water and the aqueous layer was extracted with ethyl acetate. The organic layer was dried over anhydrous sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by silica gel column chromatography (ethyl acetate:hexane = 0:100 - 50:50) to give 2 as a white solid (305 mg, 93% yield).
The reaction mixture at the first step was monitored by TLC (hexane:ethyl acetate = 1:19, Rf = 0.34).
The reaction mixture at the second step was monitored by TLC (hexane:ethyl acetate = 1:1, Rf = 0.26).
2,4,6-Trichlorophenyl 9H-Fluorene-2-carboxylate (1)
(9H-Fluoren-2-yl)(morpholino)methanone (2)
1H NMR (400 MHz, CDCl3);
(1) δ 8.41 (s, 1H), 8.29 (d, J = 8.1 Hz, 1H), 7.94-7.88 (m, 2H), 7.62 (d, J = 6.2 Hz, 1H), 7.48–7.38 (m, 4H), 4.02 (s, 2H).
(2) δ 7.81 (d, J = 7.6 Hz, 2H), 7.62-7.56 (m, 2H), 7.43-7.32 (m, 3H), 3.94 (s, 2H), 3.72 (brs, 8H).
Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.

De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.