Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Gelieve het aantal te selecteren
CAS RN: 603-35-0 | Producten #: T0519
Triphenylphosphine

Zuiverheid: >95.0%(T)
Synoniemen
- TPP
Productdocumenten:
| Afmeting | Eenheidsprijs | België | Japan* | Hoeveelheid |
|---|---|---|---|---|
| 25G |
€17.00
|
17 | ≥100 |
|
| 100G |
€34.00
|
11 | ≥80 |
|
| 500G |
€71.00
|
24 | ≥80 |
|
*Stock beschikbaar uit voorraad in België leverbaar in 1 tot 3 dagen
*stock beschikbaar uit voorraad in Japan leverbaar in 1 tot 2 weken (met uitzondering van gereguleerde producten en zendingen met droog ijs)
| Artikel # | T0519 |
| Zuiverheid / Analysemethode | >95.0%(T) |
| Moleculaire formule / molecuulgewicht | C__1__8H__1__5P = 262.29 |
| Fysieke toestand (20 graden C) | Solid |
| Opslag condities | Room Temperature (Recommended in a cool and dark place, <15°C) |
| CAS RN | 603-35-0 |
| Reaxys registratienummer | 610776 |
| PubChem product ID | 87576446 |
| SDBS | 2967 |
| Merck-index (14) | 9743 |
| MDL-nummer | MFCD00003043 |
Specificatie
| Appearance | White powder to lump |
| Purity(Iodometric Titration) | min. 95.0 % |
| Melting point | 80.0 to 82.0 °C |
| Solubility in Toluene | very faint turbidity |
| NMR | confirm to structure |
eigenschappen
| Smeltpunt | 81 °C |
| Kookpunt | 260 °C/25 mmHg |
| oplosbaarheid in water | Insoluble |
| Graad van oplosbaarheid in water | 0.3 mg/l 25 °C |
| Oplosbaarheid (oplosbaar in) | Chloroform, Benzene, Toluene |
| Oplosbaarheid (licht opl. in) | Alcohol |
GHS
| Pictogram |


|
| Signaalwoord | Gevaar |
| Gevarenaanduidingen | H302 : Schadelijk bij inslikken. H315 : Veroorzaakt huidirritatie. H319 : Veroorzaakt ernstige oogirritatie. H372 : Veroorzaakt schade aan organen bij langdurige of herhaalde blootstelling. H373 : Kan schade aan organen veroorzaken bij langdurige of herhaalde blootstelling. H317 : Kan een allergische huidreactie veroorzaken. H335 : Kan irritatie van de luchtwegen veroorzaken. H413 : Kan langdurige schadelijke gevolgen voor in het water levende organismen hebben. |
| Voorzorgsmaatregelen | P273 : Voorkom lozing in het milieu. P260 : Stof niet inademen. P264 : Na het werken met dit product de huid grondig wassen. P280 : Beschermende handschoenen/ oogbescherming/ gelaatsbescherming dragen. P314 : Bij onwel voelen een arts raadplegen. P304 + P340 + P312 : NA INADEMING: de persoon in de frisse lucht brengen en ervoor zorgen dat deze gemakkelijk kan ademen. Bij onwel voelen een ANTIGIFCENTRUM/arts raadplegen. |
Gerelateerde wetten:
| EC-nummers | 210-036-0 |
| RTECS # | SZ3500000 |
Transport informatie:
| HS-NR (invoer / uitvoer) (TCI-E) | 2931498090 |
Toepassing
t-Butyldimethylsilylation of Alcohols using TBS Reagents

References
- 1)A New Method for Silylation of Hydroxylic Compounds: Reaction of Phenols and Alcohols with Silanols Mediated by Diethyl Azodicarboxylate and Triphenylphosphine
- 2)Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
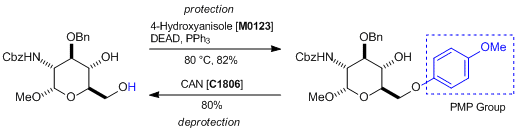
Toepassing
Protection and deprotection of PMP groups
Reference
- Protection for the Hydroxyl Group, Including 1,2- and 1,3-Diols
- P. G. M. Wuts, in Greene’s Protective Groups in Organic Synthesis, 5th ed., ed. by P. G. M. Wuts, John Wiley & Sons, Inc., Hoboken, New Jersey, 2014, Chap. 2, 17.
Toepassing
Corey-Fuchs Alkyne Synthesis

Typical Procedure:
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.
Carbon tetrabromide (34.67 g, 104.5 mmol) and anhydrous CH2Cl2 (175 mL) are added to a 500 mL round bottom flask equipped with a magnetic stir bar and septum under N2 atmosphere. The reaction flask is cooled to 0 ℃ with an ice bath for 10 min. Triphenylphosphine (54.84 g, 209.1 mmol) is added in portions over 5 min, and the dark red solution is stirred at 0 ℃ for 30 min. 3,4-Dimethoxybenzaldehyde (17.37 g, 104.5 mmol) is added and the mixture is stirred at 0 ℃ for 1 h. A 1 : 1 mixture of H2O : brine is added and the layers are separated. The aqueous layer is extracted with a 1 : 1 mixture of hexanes : CH2Cl2, and the combined organic layers are dried, filtered, and concentrated under reduced pressure. The crude material is chromatographed on SiO2 (0-15% EtOAc/hexanes) to afford the dibromide 1 (29.37 g, Y. 88%) as a yellow oil.
Then, 1 (4.41 g, 13.79 mmol, 1.0 equiv) and THF (45 mL) are added to a 250 mL round bottom flask equipped with a magnetic stir bar and septum, and the reaction flask is cooled to -78 ℃ under N2 atmosphere. 2.5M n-BuLi solution in hexane (11.6 mL) is added slowly via syringe and the reaction solution is stirred at -78 ℃ for 45 min and then at 0 ℃ for 45 min. The flask is recooled to -78 ℃, and freshly distilled methyl chloroformate (1.1 mL, 1.3 mmol, 1.0 equiv) is added slowly via syringe. The mixture is stirred at -78 ℃ for 10 min, then 0 ℃ for 1 h. Saturated aqueous NH4Cl solution is added, and the layers are separated. The aqueous layer is extracted with Et2O, and the combined organic layers are dried, filtered and concentrated under reduced pressure. The residue is chromatographed (SiO2, 2-20% EtOAc / hexanes) to afford the desired alkyne 2 (2.83 g, Y. 93%) as a white solid.
References
- Total synthesis of (+)-Lithospermic acid by asymmetric intramolecular alkylation via catalytic C-H bond activation
Toepassing
Corey-Nicolaou Macrolactonization1)

Typical Procedure:
To a stirred solution of the hydroxy carboxylic acid 1 (125 mg, 0.422 mmol) in dry CH2Cl2 (15 mL) is added triphenylphosphine (125 mg, 0.48 mmol) followed by 2,2’-dipyridyl disulfide (105 mg, 0.48 mmol) and the mixture is stirred until TLC (ethyl acetate : hexane = 2 : 3) shows complete loss of starting material (usually 0.5 h – 2 h). The mixture is concentrated in vacuo and dissolved in dry toluene (150 mL). To the solution is added 0.006M AgBF4 solution in toluene (1.2 mL) and the mixture is heated under reflux for 12 h. The mixture is cooled and solvents removed in vacuo. The residue is purified by flash column chromatography (acetone : CH2Cl2 = 0.5 : 99.5) to afford the desired lactone 2 (95 mg, Y. 81%).
To a stirred solution of the hydroxy carboxylic acid 1 (125 mg, 0.422 mmol) in dry CH2Cl2 (15 mL) is added triphenylphosphine (125 mg, 0.48 mmol) followed by 2,2’-dipyridyl disulfide (105 mg, 0.48 mmol) and the mixture is stirred until TLC (ethyl acetate : hexane = 2 : 3) shows complete loss of starting material (usually 0.5 h – 2 h). The mixture is concentrated in vacuo and dissolved in dry toluene (150 mL). To the solution is added 0.006M AgBF4 solution in toluene (1.2 mL) and the mixture is heated under reflux for 12 h. The mixture is cooled and solvents removed in vacuo. The residue is purified by flash column chromatography (acetone : CH2Cl2 = 0.5 : 99.5) to afford the desired lactone 2 (95 mg, Y. 81%).
References
- 1)Efficient and mild lactonization method for the synthesis of macrolides
- 2)Facile synthesis of saturated eight-membered ring lactones
PubMed Literatuur
Artikelen / Brochures
TCIMail
[TCIMAIL No.156] Mitsunobu Reaction Using Acetone Cyanohydrin[Product Highlights] Chiral Derivatization Reagent Possessing a Pyridylthiourea Structure
[TCI Practical Example] Bromination of an Alkyl Alcohol through the Appel Reaction
Productdocumenten (Opmerking: Voor sommige producten zijn geen analytische grafieken beschikbaar.)
Veiligheidsinformatie-blad (VIB)
Selecteer alstublieft taal.
Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Specificatiedocument
Analyse certificaat & andere certificaten
Gelieve het lotnummer in te vullen aub
Er is een onjuist lotummer ingevoerd. Voer alleen 4-5 alfanumerieke tekens vóór het koppelteken in.
Voorbeeldanalysecertificaat
Dit is een voorbeeldanalysecertificaat en vertegenwoordigt mogelijk niet een recent geproduceerde partij van het product.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.
Analytische grafieken
Gelieve het lotnummer in te vullen aub
Er is een onjuist lotummer ingevoerd. Voer alleen 4-5 alfanumerieke tekens vóór het koppelteken in.
De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.





