Maintenance Notice (4:00 AM - 9:30 AM December 13, 11:30 PM December 13 - 1:40 AM December 14 2025): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
*Voorraad beschikbaar in België: Verzending dezelfde dag
*Voorraad beschikbaar in Japan: Raadpleeg de verzendsimulatie voor geschatte levertijden. (met uitzondering van gereguleerde producten en zendingen met droogijs)
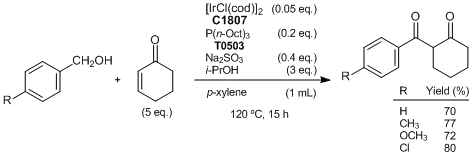
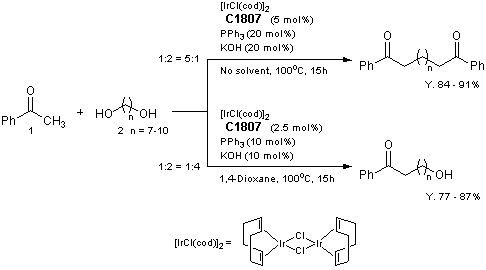
| Artikel # | C1807 |
Zuiverheid / Analysemethode 
|
>93.0%(T) |
| Moleculaire formule / molecuulgewicht | C__1__6H__2__4Cl__2Ir__2 = 671.70 |
| Fysieke toestand (20 graden C) | Solid |
Opslag condities 
|
Refrigerated (0-10°C) |
| Opslaan onder inert gas | Store under inert gas |
| Te vermijden condities | Air Sensitive,Heat Sensitive |
Verpakking 
|
1G-Glass Bottle with Plastic Insert (Bekijk afbeelding), 250MG-Glass Bottle with Plastic Insert (Bekijk afbeelding) |
| CAS RN | 12112-67-3 |
| Reaxys registratienummer | 14372408 |
| PubChem product ID | 87560730 |
| MDL-nummer | MFCD00012414 |
| Appearance | Orange to Amber to Dark red powder to crystal |
| Purity(Argentometric Titration) | min. 93.0 % |
| Smeltpunt | 205 °C(dec.) |
| oplosbaarheid in water | Insoluble |
| Pictogram |

|
| Signaalwoord | Waarschuwing |
| Gevarenaanduidingen | H315 : Veroorzaakt huidirritatie. H319 : Veroorzaakt ernstige oogirritatie. |
| Voorzorgsmaatregelen | P264 : Na het werken met dit product de huid grondig wassen. P280 : Beschermende handschoenen/ oogbescherming/ gelaatsbescherming dragen. P302 + P352 : BIJ CONTACT MET DE HUID: met veel water wassen. P337 + P313 : Bij aanhoudende oogirritatie: een arts raadplegen. P362 + P364 : Verontreinigde kleding uittrekken en wassen alvorens deze opnieuw te gebruiken. P332 + P313 : Bij huidirritatie: een arts raadplegen. |
| EC-nummers | 235-170-7 |
| HS-NR (invoer / uitvoer) (TCI-E) | 2843909000 |



Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.

De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.