Published TCIMAIL newest issue No.200
Maximum quantity allowed is 999
*Voorraad beschikbaar in België: Verzending dezelfde dag
*Voorraad beschikbaar in Japan: Raadpleeg de verzendsimulatie voor geschatte levertijden. (met uitzondering van gereguleerde producten en zendingen met droogijs)
| Artikel # | B3960 |
Zuiverheid / Analysemethode 
|
>94.0%(T) |
| Moleculaire formule / molecuulgewicht | C__6H__1__2N__2O__4S__2 = 240.29 |
| Fysieke toestand (20 graden C) | Solid |
Opslag condities 
|
Refrigerated (0-10°C) |
| Opslaan onder inert gas | Store under inert gas |
| Te vermijden condities | Air Sensitive,Heat Sensitive |
Verpakking 
|
1G-Glass Bottle with Plastic Insert (Bekijk afbeelding) |
| CAS RN | 119752-83-9 |
| Reaxys registratienummer | 21034099 |
| PubChem product ID | 354335121 |
| Appearance | White to Almost white powder to crystal |
| Purity(Iodometric back titration) | min. 94.0 % |
| Smeltpunt | 180 °C(dec.) |
| oplosbaarheid in water | Soluble |
| Pictogram |

|
| Signaalwoord | Waarschuwing |
| Gevarenaanduidingen | H315 : Veroorzaakt huidirritatie. H319 : Veroorzaakt ernstige oogirritatie. |
| Voorzorgsmaatregelen | P264 : Na het werken met dit product de huid grondig wassen. P280 : Beschermende handschoenen/ oogbescherming/ gelaatsbescherming dragen. P302 + P352 : BIJ CONTACT MET DE HUID: met veel water wassen. P337 + P313 : Bij aanhoudende oogirritatie: een arts raadplegen. P362 + P364 : Verontreinigde kleding uittrekken en wassen alvorens deze opnieuw te gebruiken. P332 + P313 : Bij huidirritatie: een arts raadplegen. |
| HS-NR (invoer / uitvoer) (TCI-E) | 2933599590 |

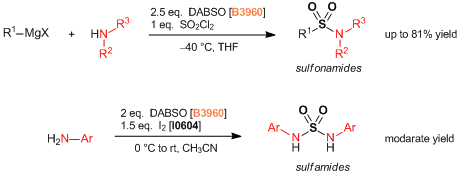
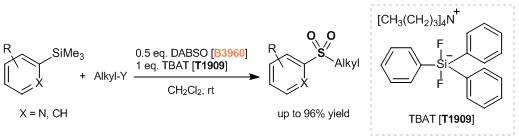
Under nitrogen atmosphere, a degassed DMI (20 mL) solution of 4-biphenylboronic acid (792 mg, 4.00 mmol), DABSO (577 mg, 2.40 mmol), tmphen (95 mg, 0.40 mmol), lithium tert-butoxide (320 mg, 4.00 mmol) and NiBr2(glyme) (123 mg, 0.40 mmol) was stirred at 100 °C for 14 hours. The mixture was cooled to room temperature and tert-butyl bromoacetate (1.17 mL, 8.00 mmol) was added and the reaction mixture stirred for 1 hour. Water (20 mL) was poured into the reaction mixture and the mixture was extracted with diethyl ether (50 mL x 3). The combined organic phase was washed with water (50 mL) and brine (40 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 5:95 - 15:85 on silica gel) to give compound 1 as a white solid (1.01 g, 76%).
NiBr2(glyme) and LiOtBu were weighed into a sample tube in a glove box because these reagents are moisture sensitive. If these were used in air, it should be weighted quickly and used up the reagents as soon as possible after opening.
Since the ligand of NiBr2(glyme) may be desorbed under reduced pressure, depressurization operations is desirable to be finished as soon as possible.
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 2:1, Rf = 0.38) and UPLC-MS.
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.00 (d, 2H, J = 8.9 Hz), 7.78 (m, 2H), 7.58–7.66 (m, 2H), 7.42-7.54 (m, 3H), 4.08 (s, 2H), 1.58 (s, 2H), 1.39 (s, 9H).

To a solution of 4-bromobiphenyl (1.17 g, 5.00 mmol), DABSO (721 mg, 3.00 mmol), and PdCl2(Amphos)2 (177 mg, 0.25 mmol) in anhydrous isopropyl alcohol (20 mL) was added triethylamine (2.1 mL, 15 mmol) at room temperature and the mixture was stirred at 75 °C for 23 hours. The suspension was cooled at room temperature and NFSI (2.36 g, 7.5 mmol) was added and the reaction mixture stirred for 3 hours until completion. The solvent was removed under reduced pressure. The residue was diluted with ethyl acetate (20 mL) and filtrated through celite pad. The filtrate was washed with saturated aqueous Na2S2O3 solution (20 mL) and brine (20 mL), dried over sodium sulfate and filtered. The solvent was removed under reduced pressure and the residue was purified by medium pressure preparative chromatography (ethyl acetate:hexane = 2:98 - 5:95 on silica gel) to give compound 1 as a white solid (928 mg, 79%).
The reaction mixture was monitored by TLC (ethyl acetate:hexane = 1:9, Rf = 0.57) and UPLC-MS.
Compound 1
1H NMR (400 MHz, CDCl3); δ 8.12–8.04 (m, 2H), 7.86–7.80 (m, 2H), 7.67–7.58 (m, 2H), 7.44-7.55 (m, 3H).

Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.

De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.