Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
CAS RN: 32005-36-0 | Producten #: B1374
Bis(dibenzylideneacetone)palladium(0)

Zuiverheid:
- Palladium(0) Bis(dibenzylideneacetone)
- Bis(dba)palladium(0)
- Pd(dba)2
| Afmeting | Eenheidsprijs | België | Japan* | Hoeveelheid |
|---|---|---|---|---|
| 1G |
€126.00
|
14 | ≥80 |
|
| 5G |
€505.00
|
6 | ≥100 |
|
*Stock beschikbaar uit voorraad in België leverbaar in 1 tot 3 dagen
*stock beschikbaar uit voorraad in Japan leverbaar in 1 tot 2 weken (met uitzondering van gereguleerde producten en zendingen met droog ijs)
| Artikel # | B1374 |
| Moleculaire formule / molecuulgewicht | C__3__4H__2__8O__2Pd = 575.02 |
| Fysieke toestand (20 graden C) | Solid |
| Opslag condities | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Opslaan onder inert gas | Store under inert gas |
| Te vermijden condities | Air Sensitive |
| CAS RN | 32005-36-0 |
| PubChem product ID | 87564299 |
| SDBS | 50493 |
| MDL-nummer | MFCD00051942 |
| Appearance | Brown to Black powder to crystal |
| Content (Palladium) | 16.5 to 20.5 % |
| Smeltpunt | 150 °C |
| Pictogram |

|
| Signaalwoord | Waarschuwing |
| Gevarenaanduidingen | H302 + H312 + H332 : Schadelijk bij inslikken, bij contact met de huid en bij inademing. H315 : Veroorzaakt huidirritatie. H319 : Veroorzaakt ernstige oogirritatie. |
| Voorzorgsmaatregelen | P261 : Inademing van stof vermijden. P264 : Na het werken met dit product de huid grondig wassen. P280 : Draag veiligheidshandschoenen/beschermende kleding/ veiligheidsbril/ gezichtsbescherming/ gehoorbeschermers. P337 + P313 : Bij aanhoudende oogirritatie: een arts raadplegen. P302 + P352 + P312 : BIJ CONTACT MET DE HUID: met veel water wassen. Bij onwel voelen een ANTIGIFCENTRUM/arts raadplegen. P304 + P340 + P312 : NA INADEMING: de persoon in de frisse lucht brengen en ervoor zorgen dat deze gemakkelijk kan ademen. Bij onwel voelen een ANTIGIFCENTRUM/arts raadplegen. |
| HS-NR (invoer / uitvoer) (TCI-E) | 2843909000 |
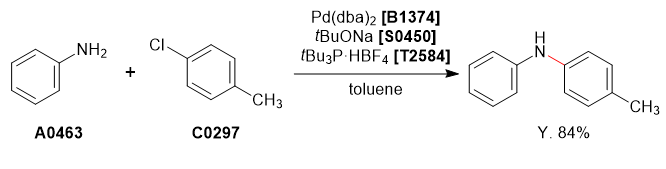
Used Chemicals
Procedure
Aniline (1.0 g, 10.7 mmol) and 4-chlorotoluene (1.4 g, 11.8 mmol) were dissolved in degassed toluene (10 mL) under nitrogen atmosphere. To this solution was added sodium tert-butoxide (1.54 g, 16.1 mmol), tri-tert-butylphosphonium tetrafluoroborate (31.1 mg, 0.1 mmol) and Pd(dba)2 (61.7 mg, 0.1 mmol, 1.5mol%). The reaction mixture was refluxed for 24 h and cooled to room temperature and quenched with H2O (10 mL). The organic phase was separated and washed with brine (10 mL), dried over MgSO4 (10 g), concentrated under reduced pressure to afford the crude product as a brown liquid. The crude product was purified by column chromatography on silica-gel (hexane : ethyl acetate = 95 : 5) to afford the desired product as a pale yellow crystalline solid (1.21 g, 6.6 mmol, 62%).
Experimenter’s Comments
Toluene was degassed by bubbling with N2 gas for 30 min.
The reaction mixture was analyzed by TLC (hexane : ethyl acetate = 1 : 1, Rf = 0.30) and GC.
Analytical Data (4-Methyl-N-phenylaniline)
1H NMR (400 MHz, CDCl3); δ 7.24-7.20 (t, J = 8.7 Hz, 3H), 7.07 (d, J = 8.7 Hz, 2H), 7.00-6.88 (m, 4H), 6.88-6.84 (m, 1H), 5.58 (s, 1H), 2.29 (s, 3H).
Lead Reference
- Tetramethoxybenzene is a Good Building Block for Molecular Wires: Insights from Photoinduced Electron Transfer
Used Chemicals
- 2-Chloro-m-xylene [C2164]
- 2-Methylphenylboronic Acid [M1313]
- Bis(dibenzylideneacetone)palladium (0) (Pd(dba)2) [B1374]
- 2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl (SPhos) [D5036]
- Tripotassium Phosphate (K3PO4)
- Toluene
- Ion-exchange water
Procedure
To a 3-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (66 mg, 0.115 mmol, 1.5 mol%), SPhos (94 mg, 0.229 mmol, 3.0 mol%), 2-methylphenylboronic acid (1.56 g, 11.5 mmol, 1.5 equiv.), tripotassium phosphate (4.87 g, 22.9 mmol, 3.0 equiv.) degassed toluene (15 mL), and ion-exchange water (1.5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 2-Chloro-m-xylene (1.0 mL, 7.64 mmol, 1.0 equiv.) was added one portion. The resulting mixture was stirred at reflux for 7 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane only) to afford the corresponding compound as a colorless oil (1.46 g, 97%).
Experimenter's Comments
i) Toluene was degassed by bubbling with nitrogen gas for 30 min.
ii) The reaction mixture was monitored by GC.Analytical Data(2,2',6-Trimethyl-1,1'-biphenyl)
1H NMR (400 MHz, CDCl3); δ 7.23-7.33 (m, 3H), 7.22-7.10 (m, 3H), 7.00-7.06 (m, 1H), 1.99 (s, 3H), 1.97 (s, 6H).
13C NMR (101 MHz, CDCl3); δ 141.0, 140.5, 135.8, 135.5, 129.9, 128.8, 127.2 127.0, 126.9, 126.0, 20.3, 19.4.
Lead Reference
- Catalysts for Suzuki−Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure
Used Chemicals
Procedure
-
To a 2-necked flask was charged with bis(dibenzylideneacetone)palladium (0) (36 mg, 0.0633 mmol, 1.5 mol%), XPhos (60 mg, 0.127 mmol, 3.0 mol%), sodium tert-butoxide (811 mg, 8.44 mmol, 2.0 equiv.) and toluene (5 mL) under nitrogen atmosphere. The mixture was stirred at room temperature for 5 min. 4-chlorotoluene (0.5 mL, 4.22 mmol, 1.0 equiv.), and morpholine (0.55 mL, 6.33 mmol, 1.5 equiv.) were added in one portion. The resulting mixture was stirred at reflux for 6 h. The reaction mixture was cooled to room temperature and quenched with water (10 mL). The organic layer was washed with water (10 mL), and brine (10 mL), dried with Na2SO4 (20 g) and then concentrated under reduced pressure. The crude product was purified by column chromatography on silica gel (hexane : ethyl acetate = 9 : 1) to afford the corresponding compound as an orange solid (700 mg, 94%).
Experimenter's Comments
Toluene was degassed by bubbling with nitrogen gas for 30 min.
The reaction mixture was monitored by GC.Analytical Data(Compound 1)
1H NMR (400 MHz, CDCl3); δ 7.10 (d, J = 8.7 Hz, 2H), 6.84 (d, J = 8.7 Hz, 2H), 3.87 (t, J = 4.8 Hz, 4H), 3.11 (t, J = 4.8 Hz, 4H), 2.28 (s, 3H).
13C NMR (101 MHz, CDCl3); δ 149.1, 129.7, 129.5, 116.0, 66.9, 49.9, 20.4.
Lead Reference
- Expanding Pd-Catalyzed C−N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions
- Pd-Catalyzed Kumada-Corriu Cross-Coupling Reactions at Low Temperatures Allow the Use of Knochel-type Grignard Reagents


References
Artikelen / Brochures
Veiligheidsinformatie-blad (VIB)
Het gevraagde SDS is niet beschikbaar.
Neem contact met ons op voor meer informatie.
Specificatiedocument
Analyse certificaat & andere certificaten
Voorbeeldanalysecertificaat
Een voorbeeldanalysecertificaat voor dit product is op dit moment niet beschikbaar.
Analytische grafieken
De gevraagde analytische grafiek is niet beschikbaar. Onze excuses voor het ongemak.







![(4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate (4,4'-Di-tert-butyl-2,2'-bipyridine)bis[3,5-difluoro-2-[5-trifluoromethyl-2-pyridinyl-κN)phenyl-κC]iridium(III) Hexafluorophosphate](/medias/D5817.jpg?context=bWFzdGVyfHJvb3R8MjU2NzV8aW1hZ2UvanBlZ3xhRGxtTDJnMU5TODVNVEUyT0RJMk1qTTVNREEyTDBRMU9ERTNMbXB3Wnd8YmJkMWY4YTBjMWZkYTI1ZDRhZTVkNzRmZTk3YzM3N2FiZTYzNDUwNGJlNzEzMjJlODIzZDUyMzA0NWNiNThjMQ)

