(1) Cycloparaphenylenes (CPP)
Carbon nanotubes (CNT) have advanced chemistry, material science, life science and other research fields. CNTs can be prepared by physical methods such as arc discharge, laser furnace, and chemical vapor deposition techniques. One disadvantage of these physical methods is forming several kinds of CNTs with various diameters, thus uniform CNTs do not form.
Cycloparaphenylenes (CPP), the so-called carbon nanoring, have a cyclic structure formed by linkages of p-substituted benzenes. The CPP attracted researchers in fundamental chemistry and material science, because it is a unit structure of CNT. In fact, Itami et al. successfully synthesized uniform CNTs by a bottom-up procedure starting from CPP as a template compound.1)
Recently, this research was extended to synthesize a CPP of smaller diameters having a large distortion. Yamago2) and Jasti3) groups independently reported synthesis of the [5]CPP, which has been the smallest CPP so far. We can expect to synthesize the smallest diameter CNTs from [5]CPP. In addition, the electronic and physical properties of [5]CPP may be interesting, because [5]CPP is a unit structure of C60 fullerene, too. CPPs of a specified diameter make an inclusion complex with a fullerene.4)
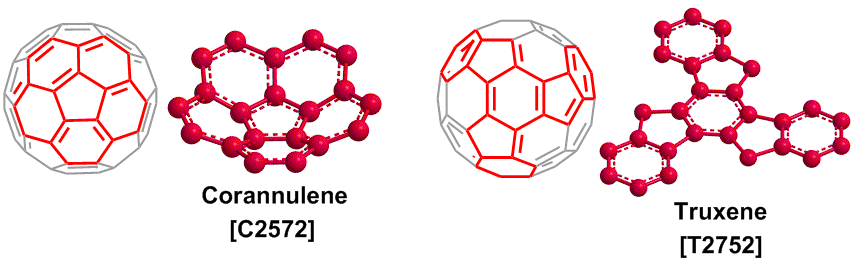
(2) Corannulene and truxene
Corannulene, the so-called [5]circulene, is one of the polycyclic aromatic compounds. It has a condensed structure of five benzene rings. The corannulene was first synthesized in 1960s,5) and after that the bowl-type structure was observed.6) The corannulene is attractive as a nanocarbon material, because it is a unit structure of C60 fullerene.
Scott et al. synthesized a polyarene compound by a flash vacuum pyrolysis (FVP) starting from corannulene. This polyarene compound e corresponds to an end-capped CNT. An extension of the end-capped CNT may chemically produce a normal CNT.7) Itami and Scott et al. synthesized a grossly warped nanographene compound from corannulene.8)

Truxene has star-shaped and rigid planar structures. Truxene is also a unit structure of C60 fullerene. Truxene derivatives are useful for OLED materials because they easily form an amorphous structure.9) A chemical synthesis for C60 fullerene was carried out starting from truxene. Otero et al. synthesized a polyarene compound formulated as C60H30 by three step reactions, and a thermal treatment of the polyarene on platinum surface gave C60 fullerene all.10)
(3) Coronene
Coronene, the so-called [6]circulene, is another polycyclic aromatic compound. It has a condensed structure of six benzene rings. The coronene is a molecular compound in nanoscale, known as a representative nanographene compound that is smaller than graphene. The coronene can be an organic transistor material11) because it is a nanographene compound with a band gap, which is different from graphene. Furthermore, a bottom-up procedure of coronene fabricated a graphene nanostructure.12) After Kubozono et al. observed superconductivity from an alkali-doped picene,13) studies on organic superconductors of polycyclic aromatic compounds recently received much attention.14,15) An alkali-doped coronene also showed superconductivity.16)

References
- 1) H. Omachi, T. Nakayama, E. Takahashi, Y. Segawa, K. Itami, Nat. Chem. 2013, 5, 572.

- 2) E. Kayahara, V. K. Patel, S. Yamago, J. Am. Chem. Soc. 2014, 136, 2284.

- 3) P. J. Evans, E. R. Darzi, R. Jasti, Nat. Chem. 2014, 6, 404.

- 4) T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino, S. Yamago, Angew. Chem. Int. Ed. 2011, 50, 8342.

- 5) W. E. Barth, R. G. Lawton, J. Am. Chem. Soc. 1966, 88, 380.

- 6) J. C. Hanson, C. E. Nordman, Acta Cryst. 1976, B32, 1147.

- 7) L. T. Scott, E. A. Jackson, Q. Zhang, B. D. Steinberg, M. Bancu, B. Li, J. Am. Chem. Soc. 2012, 134, 107.

- 8) K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott, K. Itami, Nat. Chem. 2013, 5, 739.

- 9) Z. Yang, B. Xu, J. He, L. Xue, Q. Guo, H. Xia, W. Tian, Org. Electron. 2009, ,10, 954.

- 10) G. Otero, G. Biddau, C. Sánchez-Sánchez, R. Caillard, M. F. López, C. Rogero, F. J. Palomares, N. Cabello, M. A. Basanta, J. Ortega, J. Méndez, A. M. Echavarren, R. Pérez, B. Gómez-Lor, J. A. Martín-Gago, Nature 2008, 454, 865.

- 11) I. Diez-Perez, Z. Li, J. Hihath, J. Li, C. Zhang, X. Yang, L. Zang, Y. Dai, X. Feng, K. Muellen, N. Tao, Nat. Commun. 2010, 1, 1.

- 12) X. Wan, K. Chen, D. Liu, J. Chen, Q. Miao, J. Xu, Chem. Mater. 2012, 24, 3906.

- 13) R. Mitsuhashi, Y. Suzuki, Y. Yamanari, H. Mitamura, T. Kambe, N. Ikeda, H. Okamoto, A. Fujiwara, M. Yamaji, N. Kawasaki, Y. Maniwa, Y. Kubozono, Nature 2010, 464, 76.

- 14) X. F. Wang, R. H. Liu, Z. Gui, Y. L. Xie, Y. J. Yan, J. J. Ying, X. G. Luo, X. H. Chen, Nat. Commun. 2011, 2, 1513/1.

- 15) M. Xue, T. Cao, D. Wang, Y. Wu, H. Yang, X. Dong, J. He, F. Li, G. F. Chen, Sci. Rep. 2012, 2, srep00389.

- 16) Y. Kubozono, H. Mitamura, X. Lee, X. He, Y. Yamanari, H. Takahashi, Y. Suzuki, Y. Kaji, R. Eguchi, K. Akaike, T. Kambe, H. Okamoto, A. Fujiwara, T. Kato, T. Kosugi, H. Aoki, Phys. Chem. Chem. Phys. 2011, 13, 16476.