Haruhiko Taguchi
Tokyo Chemical Industry, Co. Ltd.
In this issue of TCI MAIL, the highlighted topic of synthetic methods is dethioacetalization using
oxidizing reagents. Professor Takeda has written of the attractive usages of thioacetals, in particular, the
preparation of titanium-carbene complexes using thioacetals (see
TCIMAIL No.157 Science “spring” seminar). However, there are some serious problems to the
use of thioacetals for synthesis, even though they are very useful as building blocks. What are problems? Nasty
smell! Of course smells are a problem, but there are other problems that are of greater consequence.
It is well known that thioacetals are used as formyl anion equivalents and Prof. Seebach
originally introduced this concept of umpolung.1) This idea was very innovative because the carbonyl
carbon has an electrophilic character and it acts as a carbocation. Using the concept of umpolung, if the carbonyl
group is transformed to the corresponding thioacetal, the character of the carbon changes to a carbanion. And
furthermore, treatment of the thioacetal with a strong base generates an anionic species, which reacts with
various electrophiles. It is very easy to introduce various electrophiles into a carbonyl carbon after the
transformation of the carbonyl group.
The concept of "Umpolung" proposed by Prof. Seebach
Well now, the important question before us, is how to undo this transformation of the carbonyl to
the thioacetal? According to Seebach’s umpolung strategy, the method for hydrolysis of thioacetals, has been the
use of mercury salts. In 60’s and 70’s, mercury salts had been useful reagents in organic synthesis, but in recent
years, environmental concerns have almost completely eliminated there use in the laboratory. Almost all
laboratories do not use mercury salts in organic synthesis because chemists tend to avoid their use for
environmental reasons.
To resolve the problems associated with the use of mercury, other methods for the hydrolysis of
thioacetals have to be developed. Thioacetals are similar to acetals, so can they be hydrolyzed by the treatment
with a strong acid? This strategy is effective for the hydrolysis of acetals; however, in the case of thioacetals,
this method is not effective. The pKa value of alcohols are about 16, while that of thiols are
between 10 and 11. Since, the pKa values of thiols are 5 units higher than those of alcohols,
protonation of sulfur atom does not occur and dethioacetalizations does not proceed.
Why is mercury so effective in dethioacetalization? The reason why mercury salts have been so
effective has to do with the chemical affinity of mercury and sulfur atoms. Why is their affinity for each other
so good? It is based on the principle of hard and soft acids and bases, that is the HSAB theory, which provides
the answer.
According to HSAB theory, sulfur atoms tends to form ion pairs with soft ions, and it turns out
that mercury is a soft ion. The chemical affinity of sulfur and mercury ions is quite strong. This effect enables
mercury to form a coordinate bond with sulfur, which activates the carbon-sulfur bond towards
hydrolysis.2)
Can thioacetals be hydrolyzed by using some other soft substance? Another concept of hydrolysis is
to form sulfonium salts. Sulfur compounds, such as sulfides and sulfoxides, react readily with methyl iodide to
form methylsulfonium salts. This chemical property is specific to sulfur and oxygen does not react in this way.
The formed sulfonium salts have a positive charge and this species can undergo various reactions. By using this
technique, the hydrolysis of thioacetals can proceed. In some references, relatively strong alkylating reagents
are used to form sulfonium salts, which on subsequent hydrolysis under alkaline conditions regenerate the carbonyl
compound.3) Interestingly, this type of hydrolysis proceeds using cupper salts,4) which are
soft ions.
These synthetic techniques provide alternate methods to hydrolyze thioacetals, avoiding the use of
mercury salt. It should be noted, that there are problems associated with this method also. For one, it is often
necessary to use a strong alkylating reagent, and because these reagents have cancer inducing properties, many
chemists do not use them.
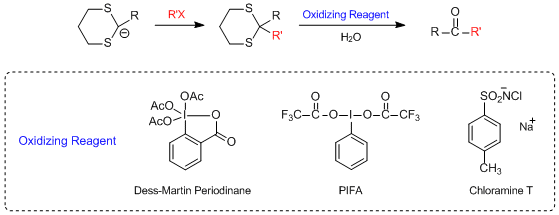
Another idea for thioacetal hydrolysis is to use oxidizing reagent. Hypervalent iodine compounds,
such as Dess-Martin periodinane,5) [Bis(trifluoroacetoxy)iodo]benzene (PIFA)6) and
2-iodoxybenzoic acid (IBX)7), are used for thioacetal hydrolysis. These oxidizing reagents oxidize the
thioacetals to sulfoxides or sulfones, and after oxidation, water-mediated hydrolysis is possible.8) If
hydrolysis is performed in an alcohol base, the formed carbonyl compound reacts with alcohols to form
corresponding acetals. Usually, acetals cannot be derived from thioacetals under acidic conditions, so this result
is potentially useful.
Dethioacetalization by oxidation with IBX is quite structure specific. IBX oxidation of benzyl or
allylthioacetals proceed rapidly, while IBX oxidation of other alkyl group thioacetals proceed slowly or not at
all.
Examples of dethioacetalizations using oxidizing reagents
Why is the hydrolysis of thioacetals possible using hypervalent iodine? Iodine is a soft ion
species, based on the HSAB theory, and thus it readily reacts with sulfur to cause oxidation.
Another unique dethioacetalization is presented. Through the use of chloramine T as an oxidizing
reagent.9) the hydrolysis of a sensitive silyl thioacetal has been reported to yield a silyl
ketone.10) Now various acylsilanes are synthesized by this method.
Synthesis of acylsilanes using Chloramine T
Chemical syntheses using thioacetals is a powerful tool for the construction of complex compounds.
TCI has many thioacetals and related reagents for making thioacetals and removal of the thioacetal group. The
Umpolung technique can be made possible by using a number of TCI reagents.
References
- 1) D. Seebach, Angew. Chem. Int.
Ed. 1979, 18, 239.

- 2) D. Seebach, A. K. Beck, Org. Synth. 1971, 51, 76.
- 3) T. Oishi, K. Kamemoto, Y. Ban,
Tetrahedron Lett. 1972, 13, 1085.

- 4) K. Mori, H. Hashimoto, Y. Takenaka, T.
Takigawa, Synthesis 1975, 720.

- 5) N. F. Langille, L. A. Dakin, J. S. Panek,
Org. Lett. 2003, 5, 575.

- 6) G. Stork, K. Zhao,
Tetrahedron Lett. 1989, 30, 287.

- 7) Y. Wu, X. Shen, J.-H. Huang,
C.-J. Tang, H.-H. Liu, Q. Hu, Tetrahedron Lett. 2002, 43, 6443.

- 8) K. Ogura, Yuki Gosei
Kagaku Kyokaishi (J. Synth. Org. Chem. Jpn.) 1979, 37, 903.

- 9) W. F. J. Huurdeman, H. Wynberg,
D. W. Emerson, Tetrahedron Lett. 1971, 12, 3449.

- 10) H. J. Reich, E. K. Eisenhart, R. E.
Olson, M. J. Kelly, J. Am. Chem. Soc. 1986, 108, 7791.

The HSAB theory, proposed by Pearson, helps to qualitatively understand the properties of
elements. The HSAB theory describes the reactions of acids with bases and defines the chemical character of acids
and bases as “Hard” or “Soft”.
Hard acids and bases generally have short atomic radius and low polarization while soft acids and
soft bases generally have long atomic radius and high polarization. As to chemical properties, hard acids tend to
bind with hard bases and soft acids tend to bind with soft bases.
The HSAB theory is a qualitative and experimental rule and it should be considered as one of
several factors in understanding the chemical reactions. This rule is useful for approximating the nature of the
chemical reaction. In particular, in considering electrostatic properties of elements, it is important to note
that hard species tend to form ionic bonds, while soft species tend to form covalent bonds.
Iodine is a soft species having the properties that large molecule size, easily polarizable and
low in electronegativity. Generally, iodine acts as a monovalent species to form a single bond with various
elements.
It can have over eight electrons beyond the octet theory by readily extending its valence. In this
expanded valence state, is called hypervalent iodinane. It is known that trivalent and pentavalent iodines are
hypervalent iodinane, and each of their structure are pseudo trigonal bipyramidal molecular geometry and square
pyramidal molecular geometry.
These hypervalent iodine compounds show strong oxidative action because the reduced monovalent
iodines possess a more stable octet structure. These chemical properties are useful for oxidation in organic
synthesis. Dess-Martin periodinane and [bis(trifluoroacetoxy)iodo]benzene (PIFA) are typical reagents for such
purposes.