Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Merci de sélectionner la quantité
Ionic Liquids
No.118(January 2004)
- E0490
- 1-Ethyl-3-methylimidazolium Chloride
- E0543
- 1-Ethyl-3-methylimidazolium Bromide
- E0556
- 1-Ethyl-3-methylimidazolium Iodide
- E0494
- 1-Ethyl-3-methylimidazolium Trifluoromethanesulfonate
- E0496
- 1-Ethyl-3-methylimidazolium Tetrafluoroborate
- E0493
- 1-Ethyl-3-methylimidazolium Hexafluorophosphate
- B2194
- 1-n-Butyl-3-methylimidazolium Chloride
- B2193
- 1-n-Butyl-3-methylimidazolium Bromide
- B2195
- 1-n-Butyl-3-methylimidazolium Tetrafluoroborate
In recent years, environmentally-friendly reaction processes have vigorously been studied from the standpoint of green chemistry. For example, oxidation reactions with the air, or reactions in water, supercritical fluids, and fluorous solvents are cited. Most recently, ionic liquids have gained much attention as green reaction solvents for organic synthesis.
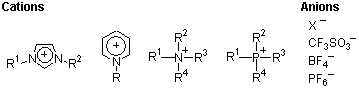
As seen above, ionic liquids are salts, consisting of cations such as imidazolium, pyridinium, quaternary ammonium and quaternary phosphonium, and anions such as halogen, triflate, tetrafluoroborate and hexafluorophosphate, which exist in the liquid state at relatively low temperatures. Their characteristic features include almost no vapor pressure, non-flammability, non-combustibility, high thermal stability, relatively low viscosity, wide temperature ranges for being liquids, and high ionic conductivity. When an ionic liquid is used as a reaction solvent, the solute is solvated by ions only, where the reaction proceeds under quite different conditions as compared to using water or ordinary organic solvents. Hence, they are expected to exhibit unconventional reactivity, and their applications in a variety of organic reactions are being explored.
Ionic liquids containing chloroaluminate as the anion have been investigated for many years. These ionic liquids are not only used as reaction solvents, but also exhibit Lewis acid or Lewis base properties, when the ratio of cations and anions is changed. However, they can only be used under an inert atmosphere or vacuum, due to their high moisture sensitivity. On the other hand, it has been found that ionic liquids containing anions such as hexafluorophosphate form stable salts in air, which lead to the synthesis of numerous stable ionic liquids today. Furthermore, some ionic liquids have very low solubility in water and polar organic solvents. Utilization of this property enables recovery and reuse of ionic liquids, after extracting the product with an organic solvent. That can help to reduce the waste of traditional solvents which are rarely reused. Moreover, ionic liquids have attracted much attention as safe solvents, due to their low volatility.
The followings are some reaction examples using ionic liquids.
Ionic liquids containing chloroaluminate as the anion have been investigated for many years. These ionic liquids are not only used as reaction solvents, but also exhibit Lewis acid or Lewis base properties, when the ratio of cations and anions is changed. However, they can only be used under an inert atmosphere or vacuum, due to their high moisture sensitivity. On the other hand, it has been found that ionic liquids containing anions such as hexafluorophosphate form stable salts in air, which lead to the synthesis of numerous stable ionic liquids today. Furthermore, some ionic liquids have very low solubility in water and polar organic solvents. Utilization of this property enables recovery and reuse of ionic liquids, after extracting the product with an organic solvent. That can help to reduce the waste of traditional solvents which are rarely reused. Moreover, ionic liquids have attracted much attention as safe solvents, due to their low volatility.
The followings are some reaction examples using ionic liquids.
1. Diels-Alder reaction
The Diels-Alder reaction between cyclopentadiene and methyl acrylate ester has been reported. In the Diels-Alder reaction using 1-ethyl-3-methylimidazolium chloride / chloroaluminate [emimCl/(AlCl3)x], the endo/exo ratio of the products varies largely, depending on the ratio of emimCl/(AlCl3)x. The amount of endo-form increases four-fold with the acidic emimCl/(AlCl3)x, compared to that of the basic emimCl/(AlCl3)x.1a) When the same reaction is carried out with 1-butyl-3-methylimidazolium tetrafluoroborate (bmimBF4), it showed similar reactivity to Lewis basic emimCl/(AlCl3)x.1b)
The Diels-Alder reaction between cyclopentadiene and methyl acrylate ester has been reported. In the Diels-Alder reaction using 1-ethyl-3-methylimidazolium chloride / chloroaluminate [emimCl/(AlCl3)x], the endo/exo ratio of the products varies largely, depending on the ratio of emimCl/(AlCl3)x. The amount of endo-form increases four-fold with the acidic emimCl/(AlCl3)x, compared to that of the basic emimCl/(AlCl3)x.1a) When the same reaction is carried out with 1-butyl-3-methylimidazolium tetrafluoroborate (bmimBF4), it showed similar reactivity to Lewis basic emimCl/(AlCl3)x.1b)

2. Heck reaction
In the Heck reaction using palladium catalysts, polar solvents such as DMF and acetonitrile are employed, and aryl iodides are normally used as substrates. In cases where the less expensive but less reactive aryl bromides or chlorides are employed, it is necessary to use more active catalysts or add phosphine ligands in order to retain the catalytic activity. By utilizing 1-butyl-3-methylimidazolium bromide (bmimBr) as solvent, aryl bromides react with styrene to afford stilbenes in high yields without adding a phosphine ligand.2a)
In the Heck reaction using palladium catalysts, polar solvents such as DMF and acetonitrile are employed, and aryl iodides are normally used as substrates. In cases where the less expensive but less reactive aryl bromides or chlorides are employed, it is necessary to use more active catalysts or add phosphine ligands in order to retain the catalytic activity. By utilizing 1-butyl-3-methylimidazolium bromide (bmimBr) as solvent, aryl bromides react with styrene to afford stilbenes in high yields without adding a phosphine ligand.2a)
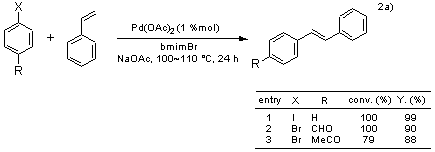
The reaction of enol ethers bearing an electron donating group with aryl halides generates a mixture of α-substituents and β-substituents under the normal Heck reaction conditions. However, the reaction of vinyl ethers with aryl halides using bmimBF4 as solvent gives only α-substituents specifically.2b) In addition, the Heck reaction employing tetrabutylammonium bromide (Bu4NBr), which is a quaternary ammonium salt, has been reported.2c)

3. Aldol condensation
The Aldol condensation reaction using ionic liquids has been reported. In the reaction for obtaining 2,4-dimethylhept-2-enal (3) from propanal via two Aldol condensations, the conversion values of the ionic liquid phase is comparable to water medium in the Aldol I reaction. However, the product selectivity is reduced, as can be seen in the figure below. This is due to a side reaction proceeding from the high solubility of product 1 toward the ionic liquid. In contrast, in the Aldol II reaction, as compared with the reaction in water, the product selectivity in ionic liquids are increased. This is because the hydrogenated product of 1 is difficult to dissolve in water but easy in ionic liquids.3)
The Aldol condensation reaction using ionic liquids has been reported. In the reaction for obtaining 2,4-dimethylhept-2-enal (3) from propanal via two Aldol condensations, the conversion values of the ionic liquid phase is comparable to water medium in the Aldol I reaction. However, the product selectivity is reduced, as can be seen in the figure below. This is due to a side reaction proceeding from the high solubility of product 1 toward the ionic liquid. In contrast, in the Aldol II reaction, as compared with the reaction in water, the product selectivity in ionic liquids are increased. This is because the hydrogenated product of 1 is difficult to dissolve in water but easy in ionic liquids.3)

4. Suzuki-Miyaura coupling reaction
In the Suzuki-Miyaura coupling reaction where biaryls are produced from aryl halides and aryl boronic acids in the presence of a palladium catalyst and a base, the removal of the catalyst is often a problem. In the system where an ionic liquid is used as solvent, the product can be extracted with ether after the reaction is complete, with the catalyst being retained in the ionic liquid. The ionic liquid and the catalyst can then be reused as they are.4)
In the Suzuki-Miyaura coupling reaction where biaryls are produced from aryl halides and aryl boronic acids in the presence of a palladium catalyst and a base, the removal of the catalyst is often a problem. In the system where an ionic liquid is used as solvent, the product can be extracted with ether after the reaction is complete, with the catalyst being retained in the ionic liquid. The ionic liquid and the catalyst can then be reused as they are.4)

5. Wittig reaction
The Wittig reaction is a useful method for C-C double bond formation. However, the separation of the product and the by-product, triphenylphosphine oxide, is a classic problem. The separation and purification are usually carried out by crystallization or chromatography. When an ionic liquid is used as solvent, the product and phosphine oxide can be easily separated by combining an ether extraction and a toluene extraction after the reaction is complete. In addition, it is possible to efficiently reuse the ionic liquid.5)
The Wittig reaction is a useful method for C-C double bond formation. However, the separation of the product and the by-product, triphenylphosphine oxide, is a classic problem. The separation and purification are usually carried out by crystallization or chromatography. When an ionic liquid is used as solvent, the product and phosphine oxide can be easily separated by combining an ether extraction and a toluene extraction after the reaction is complete. In addition, it is possible to efficiently reuse the ionic liquid.5)
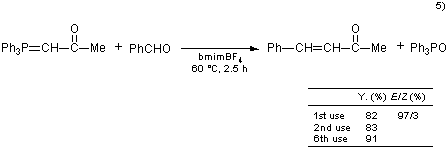
6. Stille reaction
The Stille reaction is a useful reaction, where an organotin compound and an electrophilic reagent are reacted to form a C-C bond under mild conditions in the presence of palladium catalyst. In the reaction of vinyltributyltin and iodocyclohexenone in an ionic liquid, the product can be extracted with ether, and the catalyst is retained in the ionic liquid. The ionic liquid and the catalyst can be reused as they are. This ionic liquid/catalyst phase is air and moisture stable, and thus can be used after a long storage without loss in activity.6)
The Stille reaction is a useful reaction, where an organotin compound and an electrophilic reagent are reacted to form a C-C bond under mild conditions in the presence of palladium catalyst. In the reaction of vinyltributyltin and iodocyclohexenone in an ionic liquid, the product can be extracted with ether, and the catalyst is retained in the ionic liquid. The ionic liquid and the catalyst can be reused as they are. This ionic liquid/catalyst phase is air and moisture stable, and thus can be used after a long storage without loss in activity.6)

7. Friedel-Crafts reaction
Here is an example of the Friedel-Crafts reaction. In the benzoylation of anisoles catalyzed by copper triflate in bmimBF4, methoxybenzophenone is quantitatively obtained within 1h, with a p-/o-product ratio of 96/4.7a) The same reaction performed using acetonitrile gave a lower conversion of 64% at 1h, with the reduced p-/o-product ratio of 93/7. In addition, the regioselective acylations of indoles using emimCl/(AlCl3)x has been reported as well.7b)
Here is an example of the Friedel-Crafts reaction. In the benzoylation of anisoles catalyzed by copper triflate in bmimBF4, methoxybenzophenone is quantitatively obtained within 1h, with a p-/o-product ratio of 96/4.7a) The same reaction performed using acetonitrile gave a lower conversion of 64% at 1h, with the reduced p-/o-product ratio of 93/7. In addition, the regioselective acylations of indoles using emimCl/(AlCl3)x has been reported as well.7b)

8. Hydrogenation
In the asymmetric hydrogenation of C-C double bond using homogeneous chiral transition metal complexes, the recovery of the catalyst and the seperation of the products are often troublesome.8) Dupont et al. have reported an example in which the reagents are allowed to react in a two phase system of an ionic liquid and an alcohol. After the reaction is complete, the product exists in the alcoholic phase, while the catalyst in the ionic liquid phase. Thus, the product and the catalyst can be easily separated by decantation. In addition, the catalyst which exists in the ionic liquid phase can be reused without loss in activity.
In the asymmetric hydrogenation of C-C double bond using homogeneous chiral transition metal complexes, the recovery of the catalyst and the seperation of the products are often troublesome.8) Dupont et al. have reported an example in which the reagents are allowed to react in a two phase system of an ionic liquid and an alcohol. After the reaction is complete, the product exists in the alcoholic phase, while the catalyst in the ionic liquid phase. Thus, the product and the catalyst can be easily separated by decantation. In addition, the catalyst which exists in the ionic liquid phase can be reused without loss in activity.

9. Reduction
The reduction of aldehydes using trialkylboranes is an important organic transformation reaction. However, reductions using simple trialkylboranes generally require reaction temperatures in excess of 150 dgreeC. Kabalka et al. have reported this reduction using trialkylborane in which bmimBF4, emimBF4, and 1-ethyl-3-methylimidazolium hexafluorophosphate (emimPF6) are used as solvents.9a) For example, when benzaldehyde was reduced by tributylborane in emimPF6, the reaction proceeded rapidly at 100 dgreeC to give the product in high yield. Although long reaction time is needed comparatively, the product can be obtained even at room temperature. In addition, a photoreduction has also been reported using ionic liquids.9b)
The reduction of aldehydes using trialkylboranes is an important organic transformation reaction. However, reductions using simple trialkylboranes generally require reaction temperatures in excess of 150 dgreeC. Kabalka et al. have reported this reduction using trialkylborane in which bmimBF4, emimBF4, and 1-ethyl-3-methylimidazolium hexafluorophosphate (emimPF6) are used as solvents.9a) For example, when benzaldehyde was reduced by tributylborane in emimPF6, the reaction proceeded rapidly at 100 dgreeC to give the product in high yield. Although long reaction time is needed comparatively, the product can be obtained even at room temperature. In addition, a photoreduction has also been reported using ionic liquids.9b)

10. Fluorination
The introduction of fluorines in to heterocyclic compounds is important in the synthesis of bioactive compounds. In the electrophilic fluorination of indoles using N-fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) as fluorinating agent and bmimBF4 as a solvent, 3-fluorinated 2-oxoindoles can be obtained in high yield in a short period of time compared to the conventional method (entry 1).10)
The introduction of fluorines in to heterocyclic compounds is important in the synthesis of bioactive compounds. In the electrophilic fluorination of indoles using N-fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) as fluorinating agent and bmimBF4 as a solvent, 3-fluorinated 2-oxoindoles can be obtained in high yield in a short period of time compared to the conventional method (entry 1).10)

11. Ring opening reaction
β-Aminoalcohols are utilized as useful building blocks for the synthesis of bioactive compounds. One of the synthetic methods to obtain β-aminoalcohols involves the ring opening of epoxides using amines. However, these reactions require a large excess of the amines at elevated temperatures. The high temperature reaction conditions are not only detrimental to certain functional groups but also to the control of regioselectivity. Subsequently, a variety of activators or promoters such as metal amides, metal triflates and transition metal halides have been developed. However, many of these are often expensive or are needed in stoichiometric amounts, thus limiting their practicality. In the system using ionic liquids, the reaction proceeds at room temperature to give β-aminoalcohols in high yield.11)
β-Aminoalcohols are utilized as useful building blocks for the synthesis of bioactive compounds. One of the synthetic methods to obtain β-aminoalcohols involves the ring opening of epoxides using amines. However, these reactions require a large excess of the amines at elevated temperatures. The high temperature reaction conditions are not only detrimental to certain functional groups but also to the control of regioselectivity. Subsequently, a variety of activators or promoters such as metal amides, metal triflates and transition metal halides have been developed. However, many of these are often expensive or are needed in stoichiometric amounts, thus limiting their practicality. In the system using ionic liquids, the reaction proceeds at room temperature to give β-aminoalcohols in high yield.11)

In the cases of glycidyl ether or alkyloxiranes in entries 3 and 4, amines attack on the less sterically hindered site on the epoxides. After the reaction, the product was extracted with ether, followed by drying at 80 dgreeC under reduced pressure. The ionic liquid was reused in five runs without any loss of activity.
12. Enzymatic reaction
Enzymatic reactions using ionic liquids have also been reported.12) It is known that lipase tolerates non-natural reaction conditions, and reactions in organic solvents have intensively been carried out. For example, transesterifications in organic solvents are well known as a useful synthetic methods for the preparation of optically-active compounds. In the asymmetric transesterification of allylic alcohols using ionic liquids, the desired products are afforded in similar yields to those of organic solvent systems.12a)
Enzymatic reactions using ionic liquids have also been reported.12) It is known that lipase tolerates non-natural reaction conditions, and reactions in organic solvents have intensively been carried out. For example, transesterifications in organic solvents are well known as a useful synthetic methods for the preparation of optically-active compounds. In the asymmetric transesterification of allylic alcohols using ionic liquids, the desired products are afforded in similar yields to those of organic solvent systems.12a)

As described above, a variety of reactions utilizing ionic liquids have been conducted, and the improvement of yields and the recovery and reuse of solvents have been reported. Furthermore, they are also applied to alkylations,13) allylations,14) epoxidations,15) cycloadditions,16) hydroesterifications,17) and reactions using supercritical CO2,18) in which they are reported to be effective. Ionic liquids are used not only as reaction solvents but also reported in electrochemical applications as the electrolyte of a secondary battery, due to their high ionic conductivity.
Ionic liquids are attracting attention as environmentally-friendly excellent solvents, because they are safe, easy to separate and purify from the products, recyclable as solvents, and often can be reused with the catalyst.
References
- 1)a) C. W. Lee, Tetrahedron Lett. 1999, 40, 2461.
b) T. Fischer, A. Sethi, T. Welton, J. Woolf, Tetrahedron Lett. 1999, 40, 793. - 2)a) L. Xu, W. Chen, J. Xiao, Organometallics 2000, 19, 1123.
b) L. Xu, W. Chen, J. Ross, J. Xiao, Org. Lett. 2001, 3, 295.
c) V. P. W. Bohm, W. A. Herrmann, Chem. Eur. J. 2000, 6, 1017. - 3)C. P. Mehnert, N. C. Dispenziere, R. A. Cook, Chem. Commun. 2002, 1610.
- 4)C. J. Mathews, P. J. Smith, T. Welton, Chem. Commun. 2000, 1249.
- 5)V. L. Boulaire, R. Gree, Chem. Commun. 2000, 2195.
- 6)S. T. Handy, X. Zhang, Org. Lett. 2001, 3, 233.
- 7)a) J. Ross, J. Xiao, Green Chemistry 2002, 4, 129.
b) K.-S. Yeung, M. E. Farkas, Z. Qiu, Z. Yang, Tetrahedron Lett. 2002, 43, 5793.
c) A. Stark, B. L. MacLean, R. D. Singer, J. Chem. Soc., Dalton Trans. 1999, 63.
d) C. J. Adams, M. J. Earle, G. Roberts, K. R. Seddon, Chem. Commun. 1998, 2097. - 8)A. L. Monteiro, F. K. Zinn, R. F. de Souza, J. Dupont, Tetrahedron: Asymmetry 1997, 8, 177.
- 9)a) G. W. Kabalka, R. R. Malladi, Chem. Commun. 2000, 2191.
b) J. L. Reynolds, K. R. Erdner, P. B. Jones, Org. Lett. 2002, 4, 917. - 10)a) Y. Takeuchi, T. Tarui, N. Shibata, Org. Lett. 2000, 2, 639.
b) J. Baudoux, A.-F. Salit, D. Cahard, J.-C. Plaquevent, Tetrahedron Lett. 2002, 43, 6573. - 11)J. S. Yadav, B. V. S. Reddy, A. K. Basak, A. V. Narsaiah, Tetrahedron Lett. 2003, 44, 1047.
- 12)a) T. Itoh, E. Akasaki, K. Kudo, S. Shirakami, Chem. Lett. 2001, 262.
b) R. M. Lau, F. van Rantwijk, K. R. Seddon, R. A. Sheldon, Org. Lett. 2000, 2, 4189.
c) S. Park, R. J. Kazlauskas, J. Org. Chem. 2001, 66, 8395. - 13)M. Badri, J.-J. Brunet, Tetrahedron Lett. 1992, 33, 4435.
- 14)C. M. Gordon, A. McCluskey, Chem. Commun. 1999, 1431.
- 15)G. S. Owens, M. M. Abu-Omar, Chem. Commun. 2000, 1165.
- 16)a) J. Peng, Y. Deng, New J. Chem. 2001, 25, 639.
b) J. F. Dubreuil, J. P. Bazureau, Tetrahedron Lett. 2000, 41, 7351. - 17)a) K. Qiao, Y. Deng, New J. Chem. 2002, 26, 667.
b) D. Zim, R. F. de Souza, J. Dupont, A. L. Monteiro, Tetrahedron Lett. 1998, 39, 7071. - 18)S. V. Dzyuba, R. A. Bartsch, Angew. Chem. Int. Ed. 2003, 42, 148.
- 19)Reviews
- a)T. Welton, Chem. Rev. 1999, 99, 2071.
- b)J. D. Holbrey, K. R. Seddon, Clean Prod. Proc. 1999, 1, 223.
- c)P. Wasserscheid, W. Keim, Angew. Chem. Int. Ed. 2000, 39, 3772.
- d)R. Sheldon, Chem. Commun. 2001, 2399.
- e)D. Zhao, M. Wu, Y. Kou, E. Min, Catal. Today 2002, 74, 157.
Related Compounds
- E0490
- 1-Ethyl-3-methylimidazolium Chloride (emimCl)
- E0543
- 1-Ethyl-3-methylimidazolium Bromide (emimBr)
- E0556
- 1-Ethyl-3-methylimidazolium Iodide (emiml)
- E0494
- 1-Ethyl-3-methylimidazolium Trifluoromethanesulfonate (emimOTf)
- E0496
- 1-Ethyl-3-methylimidazolium Tetrafluoroborate (emimBF4)
- E0493
- 1-Ethyl-3-methylimidazolium Hexafluorophosphate (emimPF6)
- B2194
- 1-n-Butyl-3-methylimidazolium Chloride (bmimCl)
- B2193
- 1-n-Butyl-3-methylimidazolium Bromide (bmimBr)
- B2195
- 1-n-Butyl-3-methylimidazolium Tetrafluoroborate (bmimBF4)
- E0544
- 1-Ethylpyridinium Chloride
- E0171
- 1-Ethylpyridinium Bromide
- T0055
- Tetra-n-butylammonium Chloride
- T0054
- Tetra-n-butylammonium Bromide
- T1124
- Tetra-n-butylphosphonium Bromide
- M0779
- Methyltriphenylphosphonium Bromide
- T1506
- n-Tetradecyltriphenylphosphonium Bromide
The prices are subject to change without notice. Please confirm the newest price by our online catalog before placing an order.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.
In addition, sales products changes with areas. Please understand that a product is not available when the product details page is not displayed.

