Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Merci de sélectionner la quantité
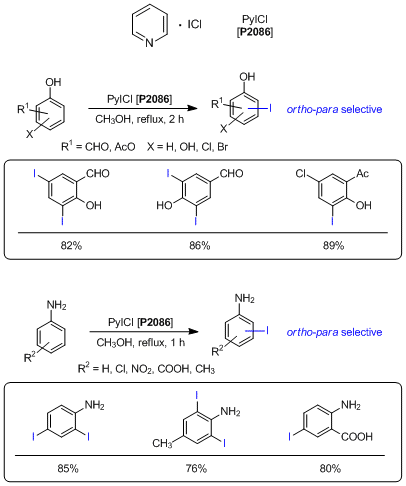
A Mild and Useful Electrophilic Iodinating Reagent
Pyridine iodine monochloride (PyICl) is a pale yellow solid and can be used as an electrophilic iodinating reagent. While iodine monochloride exists in the solid state under ambient temperature with a low melting point, PyICl is a solid compound having a melting point over 100 °"C. Its remarkable property enables it to be handled easily. Furthermore, general electrophilic iodinating reagents such as the Barluenga reagent (IPy2BF4) require excess amount of some acids to accomplish iodinations. In a case using PyICl as an iodinating reagent, acids are not required to iodinate and formyl or acetyl group substituted phenols and anilines are iodinated with ortho-para selectivity under near-neutral conditions. As described above, PyICl is expected to be used for a mild and efficient electrophilic iodinating reagent.
References
- 1)Pyridinium Iodochloride: An Efficient Reagent for Iodination of Hydroxylated Aromatic Ketones and Aldehydes
- 2)Convenient and Efficient Method for the Iodination of Aromatic Amines by Pyridinium Iodochloride

