Haruhiko Taguchi
Tokyo Chemical Industry, Co. Ltd.
In this column, we focus on another usage of reagents from the viewpoint of a reagent company. In this issue, we introduce a reagent having some probable reactive sites. In general, organic synthetic reagents have a certain reactive site and this chemical property is utilized for organic synthesis. Reagents having some active reactive sites such as dihalogen compounds and haloalcohols are available from reagent companies and these chemical properties can be used for design and synthesis of compounds with more complex structures.
However, there are some unusual reagents in organic chemistry and some of them are available from reagent companies. Now, we pick chlorosulfonyl isocyanate (CSI) and focus on its unique reactivities.
The reactivities of chlorosulfonyl isocyanate
CSI has been well used for organic syntheses due to its unique reactivities. CSI is composed of two functional groups, the isocyanate portion and the chlorosulfonyl group. In “Encyclopedia of Reagents for Organic Synthesis”, it is written that CSI has three reactive sites.1) The isocyanate portion of CSI shows two reactivities: the cycloaddition at the carbon-nitrogen double bond and the nucleophilic addition at the carbon-oxygen double bond. The chlorosulfonyl group of CSI proceeds with nucleophilic substitution. How can each reactivity of CSI be successfully controlled? In fact, these three reactivities can be successfully controlled.
The reactions using chlorosulfonyl isocyanate
We understand that the isocyanate portion is the higher reactive functional group compared with the reactivity of the chlorosulfonyl group based upon accumulated experiments. Actually, in the reactions using CSI, the isocyanate portion generally reacts prior to the chlorosulfonyl group, thereby allowing the CSI reactivities to be controlled even with having three reactive sites.
Especially, the β-lactam moiety formed by the cycloaddition of unsaturated compounds to the carbon-nitrogen double bond of CSI, is the essential part to synthesize the β-lactam antibiotics (Path A). Then, how to utilize the chlorosulfonyl group of CSI in organic synthesis? As shown in Path A, B, and C in the above scheme, there are some usages. The residual chlorosulfonyl group after the reaction of CSI with nucleophiles is available for further reactions, and one of the typical reactions of it is with other nucleophiles (Path C).
One example reaction through the Path C route is the preparation of Burgess reagent, an efficient reagent converting alcohols to the related amines or olefins, which is synthesized from CSI.3) On the other hand, other methods to use the chlorosulfonyl group of CSI like Path A or Path B are the aqueous hydrolyses.1,2)
As shown in Path A and Path B in above scheme, the chlorosulfonyl group of the intermediates is hydrolyzed by the addition of water to afford the desired N-free amines. In these reactions, we recognize that chemically same products can be obtained even when using isocyanic acid instead of CSI, and CSI can be also used as an isocyanic acid equivalent. Isocyanic acid is a highly toxic and volatile liquid with a low boiling point. Thus, CSI is an efficient reagent as an alternative to using isocyanic acid.
The usage of CSI as an isocyanic acid equivalent
As mentioned above, the reactivity of CSI is quite high. How much toxicity and chemical stability does CSI show? CSI is toxic and hygroscopic due to one of isocyanate derivatives and reacts easily with water to decompose. It seems difficult to store CSI without decomposition. If CSI is partially decomposed by moisture, will it be able to be used as a reagent anymore? In a case of CSI, the reaction shown in the scheme below occurs as hygroscopic decomposition.
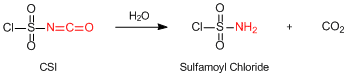
Hydrolysis of CSI to generate sulfamoyl chloride
The isocyanate portion of CSI is hydrolyzed by moisture and crystalline sulfamoyl chloride is formed. In experiments, CSI has formed some solids in a bottle and a cap with use. It has been caused by the hygroscopic decomposition of CSI to form crystalline sulfamoyl chloride. However, even when CSI is partially decomposed and crystalline sulfamoyl chloride precipitates appear in a bottle, the supernatant liquid of CSI has kept the purity sufficiently high and it can be still used as a reagent.
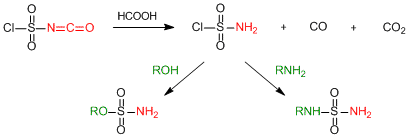
By the way, sulfamoyl chloride can be also used for a sulfonamide bond forming reaction, which enables CSI to be used as the precursor of sulfamoyl chloride. Actually, in situ preparation of sulfamoyl chloride by the treatment of CSI with water is not safe, so the same equivalent of formic acid toward CSI is better used instead of water to generate sulfamoyl chloride more gently.4)
Preparation of sulfamoyl chloride from CSI and its use for organic synthesis
When preparing sulfamoyl chloride according to above mentioned method, we find that crystalline sulfamoyl chloride is being formed as a precipitate by the decomposition of liquid CSI. The reactivities of formed sulfamoyl chloride to alcohols and amines are excellent and the desired sulfonamide derivatives are given. This synthetic manner is especially efficient to produce N-free terminal sulfonamide derivatives.
The usage of CSI as a reagent is very unique because of having three different reactive sites. In addition, it can be also used as an isocyanic acid equivalent and the precursor of sulfamoyl chloride. Furthermore, CSI would be a powerful tool to construct the sulfonamide structure which is often seen in bioactive compounds. As one method to synthesize the bioactive compounds, chlorosulfonyl isocyanate is recommended for your organic synthesis.
References
- 1)M. J. Miller, M. Ghosh, P. R. Guzzo, P. F. Vogt, J. Hu, in Encyclopedia of Reagents for Organic Synthesis, ‘Acetone Cyanohydrin’, John Wiley & Sons, 1995, 1213.
- 2)D. N. Dhar, K. S. K. Murthy, Synthesis 1986, 437.

- 3)E. M. Burgess, H. R. Penton, Jr., E. A. Taylor, W. M. Williams, Org. Synth. 1977, 56, 40.
- 4)R. Appel, G. Berger, Chem. Ber. 1958, 91, 1339.