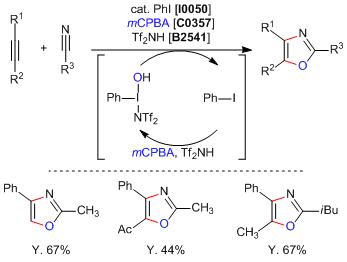
Saito
et al. have reported a metal-free synthesis of oxazole rings by a formal [2+2+1] cycloaddition. The reaction is mediated by a catalytic
in situ generated iodine (III) species to cyclize an alkyne and nitrile moieties affording di or trisubistuted oxazoles with good yield and complete regioselectivity. It was experimentally determined that the hypervalent iodine moiety is formed
in situ by the treatment of a catalytic amount of
iodobenzene with
mCPBA and
bis(trifluoromethanesulfonyl)imide (= Tf2NH). This result represents the first example of a multicomponent reaction using a hypervalent iodine (III) compound.