Published TCIMAIL newest issue No.198
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 1109-15-5 | Product Number: T2313
Tris(pentafluorophenyl)borane
Purity: >98.0%(NMR)
Synonyms:
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 1G |
€ 93,00
|
25 | ≥100 |
|
| 5G |
€ 362,00
|
10 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | T2313 |
| Purity / Analysis Method | >98.0%(NMR) |
| Molecular Formula / Molecular Weight | C18BF15 = 511.98 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Frozen (<0°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Hygroscopic,Heat Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 1109-15-5 |
| Reaxys Registry Number | 2931347 |
| PubChem Substance ID | 87558752 |
| Merck Index (14) | 9755 |
| MDL Number | MFCD00269813 |
Articles/Brochures
TCIMAIL
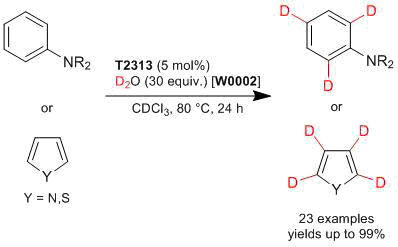
[Product Highlights] Deuteration of Aromatic and Heteroamomatic Compounds Catalyzed by Tris(pentafluorophenyl)borane[Product Highlights] A Bisphosphine Usable for Metal-free Hydrogenations
[Research Articles] High Throughput Sequence-controlled Oligosiloxane Synthesis
[Research Articles] Tri(cyclohexa-2,5-dien-1-yl)silane: A Stable and Easy-to-handle Surrogate of Monosilane (SiH4)
[Research Articles] Metal-Free Hydrogenation of Ketone by Frustrated Lewis Pairs (FLPs)
[Research Articles] Metal-Free Hydrogenation of N-Phenyl Aromatic Rings
[Research Articles] Metal-Free Hydrogenation of Imines Catalyzed by B(C6F5)3
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.