Published TCIMAIL newest issue No.196
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 18284-36-1 | Product Number: H1317
Hydridotetrakis(triphenylphosphine)rhodium(I)

Purity:
Synonyms:
- Tetrakis(triphenylphosphine)rhodium(I) Hydride
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 200MG |
€146.00
|
Contact Us | 7 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | H1317 |
| Molecular Formula / Molecular Weight | C__7__2H__6__1P__4Rh = 1,153.08 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| CAS RN | 18284-36-1 |
| PubChem Substance ID | 135727037 |
| MDL Number | MFCD00015867 |
Specifications
| Appearance | Light yellow to Brown powder to crystal |
| Rhodium Content | 8.40 to 9.40 % |
| IR | confirm to structure |
Properties (reference)
| Melting Point | 146 °C |
GHS
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
Related Laws:
Transport Information:
| HS Number | 2843909000 |
Application
Regiospecific Hydrosilation of alpha,beta-Unsaturated Carbonyl Compounds
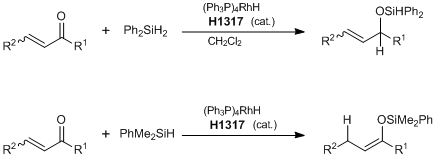
Typical Procedure: (1,2-Reduction) (Ph3P)4RhH (ca. 5 mg) is placed in a 10 mL flask furnished with a magnetic stirring bar and sealed with a rubber septum under argon. Dried CH2Cl2 (1 mL) is added via a syringe followed by the alpha,beta-unsaturated ketone (1 mmol). After 10 min, diphenylsilane (1.3 mmol) is added dropwise. The reaction mixture is stirred at room temperature for 4 h and then transferred to a 25 mL flask with a solution of HCl (2 N, 3 mL)―acetone (3 mL). The mixture is stirred at room temperature for 2 h. Acetone is removed via rotary evaporation. The aqueous residue is then extracted with dichloromethane (4 × 3 mL). The combined organic layers are dried over Na2SO4. Removed of solvent gives the crude product, which is purified by flash column chromatography separation or Kugelrohr distillation. (1,4-Reduction) In a 5 mL flask furnished with a magnetic stirring bar and sealed with a rubber septum, (Ph3P)4RhH (ca. 5 mg) is placed under argon. The alpha,beta-unsaturated ketone (1 mmol) is introduced to the flask via a syringe followed by the dimethylphenylsilane (1.1 mmol). The reaction mixture is stirred at room temperature for 12 h, and then hexane (1 mL) are added. The mixture is filtered and the solvent is removed to get the crude product, which is purified by Kugelrohr distillation.
References
- T. H. Chan, G. Z. Zheng, Tetrahedron Lett. 1993, 34, 3095.

- G. Z. Zheng, T. H. Chan, Organometallics 1995, 14, 70.

PubMed Literature
Articles/Brochures
TCIMAIL
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.





