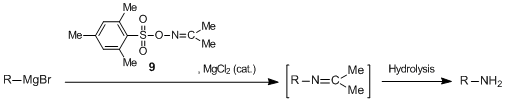The Gabriel reagent is widely used as a nucleophilic aminating agent since its reactivity is reliable and its handling is easy.1) A variety of aminating agents analogous to the Gabriel reagent have been reported.
The potassium salt of imide 1 reacts with alkyl halide to give imide 2. 2 is treated with base or acid to give either N-Boc-amine 3 or N-methoxycarbonylamine 4, respectively. Moreover, 1 also serves as an acid nucleophile in the Mitsunobu reaction to allow for the conversion of hydroxy groups to amino groups. Therefore, 1 is a useful reagent to give monoprotected amines.2)