Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
Please select the quantity
CAS RN: 81290-20-2 | Product Number: T1570
(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]
![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] No-Image](/medias/T1570.jpg?context=bWFzdGVyfHJvb3R8NzU0MTB8aW1hZ2UvanBlZ3xhRGRsTDJoaVpTODRPVEl6TkRFM05qY3pOelU0TDFReE5UY3dMbXB3Wnd8YjdiMmNiNWZiODExMzE5ZDE3Mzg5MDVmNDYwOGIzYTM1OGQxYzY1YWZhZTlkNTg3OTczMmI0MmY2YzhhNTQzZg)
Purity: >97.0%(GC)
Synonyms:
- (Trimethylsilyl)trifluoromethane
- Ruppert-Prakash Reagent
Product Documents:
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 5G |
51,00 €
|
3 | ≥60 |
|
| 25G |
162,00 €
|
6 | ≥100 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | T1570 |
| Purity / Analysis Method | >97.0%(GC) |
| Molecular Formula / Molecular Weight | C__4H__9F__3Si = 142.20 |
| Physical State (20 deg.C) | Liquid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Moisture Sensitive |
| CAS RN | 81290-20-2 |
| Reaxys Registry Number | 4241868 |
| PubChem Substance ID | 87577328 |
| SDBS (AIST Spectral DB) | 19694 |
| Merck Index (14) | 9683 |
| MDL Number | MFCD00145454 |
Specifications
| Appearance | Colorless to Light yellow clear liquid |
| Purity(GC) | min. 97.0 % |
Properties (reference)
| Boiling Point | 55 °C |
| Flash point | -32 °C |
| Specific Gravity (20/20) | 0.96 |
| Refractive Index | 1.33 |
GHS
| Pictogram |

|
| Signal Word | Danger |
| Hazard Statements | H225 : Highly flammable liquid and vapour. |
| Precautionary Statements | P501 : Dispose of contents/ container to an approved waste disposal plant. P210 : Keep away from heat, hot surfaces, sparks, open flames and other ignition sources. No smoking. P233 : Keep container tightly closed. P370 + P378 : In case of fire: Use dry sand, dry chemical or alcohol-resistant foam to extinguish. P303 + P361 + P353 : IF ON SKIN (or hair): Take off immediately all contaminated clothing. Rinse skin with water. P403 + P235 : Store in a well-ventilated place. Keep cool. |
Related Laws:
Transport Information:
| UN Number | UN1993 |
| Class | 3 |
| Packing Group | II |
| HS Number | 2931900090 |
Application
Synthesis of Trifluoromethylketones from Weinreb Amides
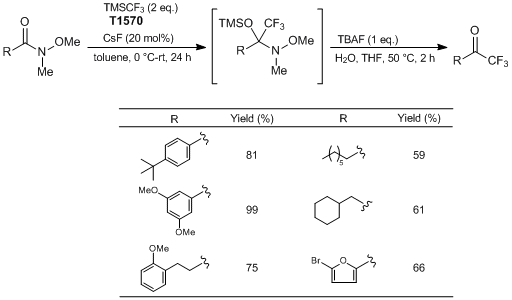
Typical Procedure:
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
To a 50 mL round bottom flask equipped with a stir bar is added CsF (0.1512 g, 1.0 mmol, 0.2 eq.). Toluene (2.5 mL) is added to the flask, followed by 4-(tert-butyl)-N-methoxy-N-methylbenzamide (1.11 g, 5.0 mmol, 1 eq.). The flask is sealed with a septum equipped with an inlet needle as an exit valve. The flask is cooled to 0 °C for 10 minutes. Once cooled, TMSCF3 (1.42 g, 10.0 mmol, 2 eq.) is added to the reaction mixture dropwise over a period of ≈ 10 minutes. After completion of addition, the reaction mixture is allowed to stir at 0 °C for 10 minutes. The cooling bath is removed and the reaction mixture is allowed to stir at room temperature overnight. (Upon reaching room temperature, the reaction occurs and is mildly exothermic and gas is evolved.) Reaction progress is monitored by 1H NMR.
Once complete conversion to the silylated intermediate is confirmed, water (5 mL) followed by TBAF (5 mL, 1 M in THF, 1 eq.) is added to the reaction flask. The flask is equipped with a reflux condenser, open to air. The contents are then heated to 50 °C, and allowed to stir at that temperature for 2 hours. Once cooled to room temperature, the reaction mixture is diluted with Et2O (≈ 30 mL), and transferred to a separatory funnel. The organic layer is washed with deionized water (3 × 30 mL), followed with a brine solution (1 × 30 mL). The organic layer is dried with Na2SO4 and the solvent is removed in vacuo by rotary evaporation to yield crude trifluoromethylketone. Further purification is accomplished by flash chromatography (hexane : EtOAc = 8 : 2) to produce the pure CF3 ketone as an orange solid (0.935 g, Y. 81%).
References
Application
Nucleophilic Trifluoromethylation

Reference
Application
Difluorocarbene Precursor

Reference
- Synthesis of gem-difluorinated cyclopropanes and cyclopropenes: trifluoromethyltrimethylsilane as a difluorocarbene source
PubMed Literature
Articles/Brochures
Product Documents (Note: Some products will not have analytical charts available.)
Safety Data Sheet (SDS)
Please select Language.
The requested SDS is not available.
Please Contact Us for more information.
Specifications
C of A & Other Certificates
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
Sample C of A
This is a sample C of A and may not represent a recently manufactured lot of the product.
A sample C of A for this product is not available at this time.
Analytical Charts
Please enter Lot Number
Incorrect Lot Number. Please input only the 4-5 alphanumeric characters before the hyphen.
The requested analytical chart is not available. Sorry for the inconvenience.



![(Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent] (Trifluoromethyl)trimethylsilane [Trifluoromethylating Reagent]](/_ui/responsive/theme-tci/images/missing_product_zoom.jpg)

