Maintenance Notice (4:30 AM - 11:00 AM December 21, 2024): This website is scheduled to be unavailable due to maintenance. We appreciate your patience and understanding.
Published TCIMAIL newest issue No.197
Maximum quantity allowed is 999
| Size | Unit Price | Belgium | Japan* | Quantity |
|---|---|---|---|---|
| 1G |
€86.00
|
Contact Us | 14 |
|
| 10G |
€264.00
|
1 | 8 |
|
*Stock available in Belgium will be delivered in 1 to 3 days
*Stock available in Japan will be delivered in 1 to 2 weeks (excludes regulated items and dry ice shipments).
| Product Number | B6316 |
| Purity / Analysis Method | >95.0%(T)(HPLC) |
| Molecular Formula / Molecular Weight | C__1__4H__1__9NO__6 = 297.31 |
| Physical State (20 deg.C) | Solid |
| Storage Temperature | Room Temperature (Recommended in a cool and dark place, <15°C) |
| Store Under Inert Gas | Store under inert gas |
| Condition to Avoid | Air Sensitive |
| Packaging and Container | 1G-Glass Bottle with Plastic Insert (View image) |
| CAS RN | 19350-66-4 |
| Reaxys Registry Number | 489397 |
| PubChem Substance ID | 468591034 |
| MDL Number | MFCD00417306 |
| Appearance | White to Light yellow powder to crystal |
| Purity(HPLC) | min. 95.0 area% |
| Purity(Neutralization titration) | min. 95.0 % |
| NMR | confirm to structure |
| Melting Point | 220 °C |
| Pictogram |

|
| Signal Word | Warning |
| Hazard Statements | H315 : Causes skin irritation. H319 : Causes serious eye irritation. |
| Precautionary Statements | P264 : Wash skin thoroughly after handling. P280 : Wear protective gloves/ eye protection/ face protection. P302 + P352 : IF ON SKIN: Wash with plenty of water. P337 + P313 : If eye irritation persists: Get medical advice/ attention. P362 + P364 : Take off contaminated clothing and wash it before reuse. P332 + P313 : If skin irritation occurs: Get medical advice/ attention. |
| HS Number | 2933399990 |
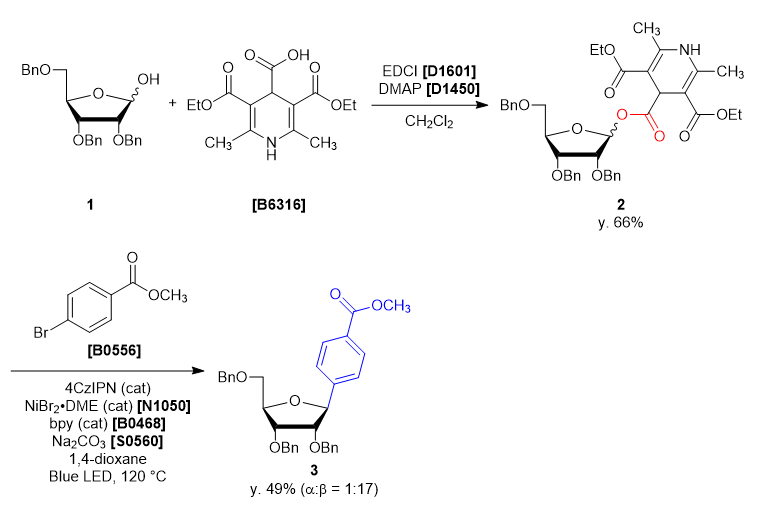
A four-neck round bottom flask was charged with 2,3,5-tri-O-benzyl-α/β-D-ribofuranose (1) (2.165 g, 5.15 mmol, 1 eq) and dichloromethane (26 mL). The solution was cooled under 5 ˚C, then DMAP (0.063 g, 0.51 mmol, 0.1 eq), EDCI (1.38 g, 7.21 mmol, 1.4 eq), 3,5-bis(ethoxycarbonyl)-2,6-dimethyl-1,4-dihydropyridine-4-carboxylic acid (1.684 g, 5.66 mmol, 1.1 eq) was added. The reaction mixture was allowed to warm to ambient temperature and stirred for 46 h. After the reaction, the reaction mixture was quenched with ion-exchanged water (35 mL). The solution was transferred into a separatory funnel, and the aqueous layer was extracted with dichloromethane (10 mL, twice). The combined organic layers were washed with brine, dried over sodium sulfate (10 g) for about 30 minutes and then filtered. The solvent was removed in vacuo, giving crude as a blown oil (3.91 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 1:1, Rf = 0.45) to give compound 2 as a light yellow oil (2.99 g, y. 66%).
30 mL sealed vessel was charged with 4CzIPN (0.0158 g, 0.020 mmol, 0.1 eq), methyl 4-bromobenzoate (0.0858 g, 0.399 mmol, 2.0 eq), sodium carbonate (0.0443 g, 0.418 mmol, 2.1 eq), 2,2’-bipyridine (0.0086 g, 0.055 mmol, 0.28 eq) at rt under N2. A solution of NiBr2・DME (0.012 g, 0.040 mmol, 0.20 eq) in 1,4-dioxane (5 mL) and the solution of 1 (0.14 g, 0.20 mmol, 1.0 eq) in 1,4-dioxane (5 mL) were added to the sealed vessel. The mixture was placed in a preheated oil bath whose temperature was set to 120 ˚C, and placed at a distance of 2-3 cm from Blue LED lamp. After irradiation for 46 h, the reaction mixture was cooled to room temperature and filtered through Celite pad (1 cm). The solvent was removed in vacuo and the crude was given as a yellow oil (0.23 g). The crude was purified by silica gel column chromatography (hexane:ethyl acetate = 10:2, Rf = 0.30) to give compound 3 as a colorless oil (0.053 g, y. 49%).
Compound 3
1H NMR (270 MHz, CDCl3); δ 7.96 (d, J = 8.2 Hz, 2H), 7.46 (d, J = 8.2 Hz, 2H), 7.40-7.30 (m, 11H), 7.26-7.23 (m, 2H), 7.18-7.15 (m, 2H), 5.05 (d, J = 6.9 Hz, 1H), 4.71-4.44 (m, 6H), 4.39-4.34 (m, 1H), 4.01 (dd, J = 5.3, 3.3 Hz, 1H), 3.92 (s, 3H), 3.77 (dd, J = 6.9, 5.3 Hz, 1H), 3.65 (qd, J = 10.6, 3.9 Hz, 2H).
The requested SDS is not available.
Please Contact Us for more information.
A sample C of A for this product is not available at this time.
The requested analytical chart is not available. Sorry for the inconvenience.
